Key Points
Brincidofovir has superior antiadenoviral activity and safety profile compared with cidofovir.
Brincidofovir is highly efficacious in controlling adenoviremia during the lymphopenic phase of HCT.
Abstract
Cidofovir is preemptively used for controlling adenoviremia and preventing disseminated viral disease in hematopoietic cell transplant (HCT) recipients but does not lead to resolution of viremia without T-cell immune-reconstitution. The lipid-conjugated prodrug of cidofovir, brincidofovir, has improved oral bioavailability and achieves higher intracellular concentrations of active drug. We present retrospective multicenter data comparing the kinetics of viremia and toxicities following preemptive treatment with and brincidofovir in children and adolescents diagnosed with HCT-related adenoviremia. Forty-one episodes (18 = brincidofovir; 23 = cidofovir) of antiviral therapy were observed in 27 patients. The 2 groups had comparable immune-reconstitution and viral burden. Major (≥2 log-reduction in 2 weeks; n = 13) and minor (≥1 to ≤2 log-reduction in 2 weeks; n = 2) virological responses were observed in 15 (83%) brincidofovir episodes compared to only 2 (9%) major virological responses with cidofovir (P < .0001). Brincidofovir mediated major responses in 9 of 11 cidofovir-unresponsive patients and resulted in complete responses (CR) despite significant lymphopenia (Brincidofovir vs cidofovir; CR = 13 (80%) vs 8 (35%); median lymphocyte count = 320/μl vs 910/μl; P < .05). One patient experienced abdominal cramps and diarrhea necessitating interruption of brincidofovir and none developed nephrotoxicity with brincidofovir. Thus, brincidofovir is well-tolerated and highly efficacious in controlling adenoviremia during the lymphopenic phase of HCT.
Introduction
There is no established preemptive therapy to control adenoviremia post-hematopoietic cell transplant (HCT), despite a 15% risk of significant viral reactivation1 and a 20% to 80% risk of mortality in disseminated disease.2 Cidofovir, a monophosphate nucleotide analog of deoxycytidine competitively inhibits incorporation of deoxycytidine into viral DNA, leading to viral DNA termination and is the only drug readily available to control HCT-related adenoviremia. Although, the use of preemptive antiviral therapy/rituximab has significantly reduced cytomegalovirus (CMV)/Epstein-Barr virus-related morbidity and mortality following HCT, cidofovir had no comparable impact on the management of adenoviral problems.3-6 Cidofovir is nephrotoxic,7 has poor cellular uptake, and is unable to mediate complete resolution of viremia in the absence of immune recovery.8,9 A lipid-linked derivative of cidofovir, brincidofovir (CMX001; Chimerix Inc), has recently been developed. The lipid conjugation improves oral bioavailability and increases intracellular concentration of the active drug. Brincidofovir, unlike cidofovir, is not a substrate for organic anion transporter 1 in the renal tubules and hence is not nephrotoxic.10 We therefore compared the efficacy of brincidofovir and cidofovir in controlling HCT-related adenoviremia and assessed the toxicity profile in a retrospective UK-wide multicenter pediatric study.
Study design
Definition of significant viremia and exclusion criteria
A total of 333 recipients undergoing allogeneic stem cell transplant in 7 pediatric transplant centers from January 2015 to May 2016 were screened weekly using an adenovirus polymerase chain reaction (PCR) until immune recovery and cessation of immunosuppression. Patients with significant viremia (adenovirus levels ≥1000 copies per mL) on 2 consecutive occasions were treated with an antiviral therapy. Patients with single PCR positivity and/or with PCR level below the limit of sensitivity were excluded. Patients gave informed consent.
Treatment of adenoviremia
Withdrawal of immunosuppression was commenced immediately in patients with significant viremia and without graft-versus-host disease (GVHD).11 The standard-of-care preemptive therapy was either cidofovir 5 mg/kg weekly for 2 consecutive weeks followed by fortnightly dosing or 1 mg/kg 3 times weekly. Patients with preexisting renal impairment, those who developed renal impairment on cidofovir, or those not responding to 2 weeks of cidofovir were considered for treatment with 2 mg/kg twice weekly brincidofovir through an expanded access program.
Assessment of virological response and its relationship with lymphocyte reconstitution
Major and minor virological responses were defined as 2-log and 1-log reduction of viral load, respectively, within 2 weeks of starting antiviral therapy. Treatment failure was defined as no reduction in viral load within 2 weeks of commencing antiviral therapy. The relationship between virological response and lymphocyte reconstitution was also studied.
The peak viral load and the total lymphocyte count at commencement of antiviral therapy were recorded (see supplemental Figure 1, available on the Blood Web site). The area under the curve (AUC) of viral load against time on therapy was recorded during antiviral therapy (supplemental Figure 2).
Toxicity profile
The Common Terminology Criteria for Adverse Events version 4.0 was used to assess the toxicity of antiviral therapy.12
Statistical analysis
Fischer’s exact test and unpaired Student t test was used to analyze categorical variables and continuous variables, respectively.
Results and discussion
Adenoviremia was reported in 47 (14.1%) patients and significant viremia requiring antiviral treatment was noted in 27 patients (8.1%). Five patients had adenoviral disease at onset of viremia. The transplant characteristics of patients with significant viremia are summarized in supplemental Table 1.
Patients were treated with cidofovir only (n = 9), cidofovir followed by brincidofovir (n = 12), brincidofovir only (n = 4), and cidofovir followed by adenovirus-specific cytotoxic lymphocytes (n = 2). A total of 18 brincidofovir episodes in 16 patients and 23 cidofovir episodes in 23 patients were observed. Thirteen brincidofovir episodes received concurrent steroid treatment of GVHD (2 mg/kg of methylprednisolone, n = 8; 1 mg/kg of methylprednisolone, n = 5) and 12 cidofovir episodes received such treatment (2 mg/kg of methylprednisolone, n = 6; 1 mg/kg of methylprednisolone, n = 6). Patients treated with cidofovir vs brincidofovir were comparable for use of serotherapy and HLA matching (supplemental Table 2). The median time from HCT to initiation of cidofovir vs brincidofovir was 28 days (interquartile range [IQR], 17 to 43) vs 53 days (IQR, 29 to 78), respectively. However, the 2 groups had comparable compromise of immune reconstitution and similar viral burden (supplemental Figure 1).
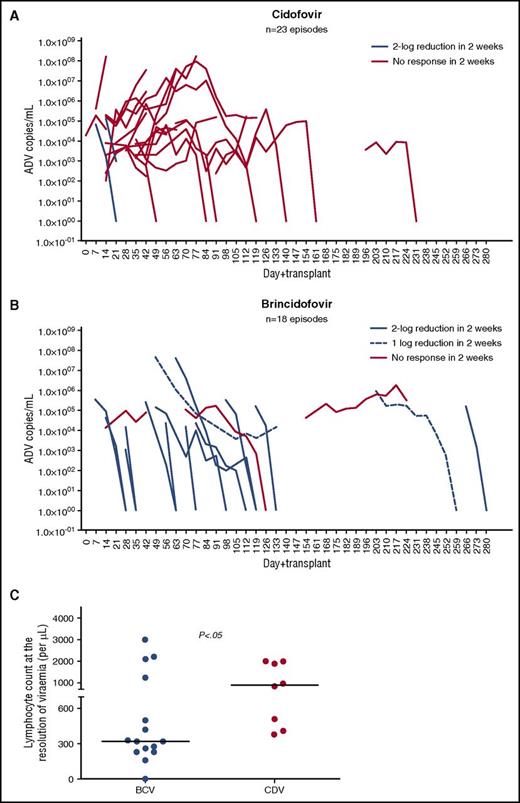
Brincidofovir mediated major and minor virological responses in 13 (72%) and 2 episodes (11%), respectively. In contrast, cidofovir mediated major virological responses only in 2 episodes (9%) (P < .0001) (Figure 1A-B, 2A and supplemental Figure 3; supplemental Table 3). Thirteen patients (80%) cleared viremia with brincidofovir compared with 8 patients (35%) with cidofovir. The median time to clearance of viremia with brincidofovir was 4 weeks (range, 2 to 9 weeks) compared with 9 weeks (range, 3 to 15 weeks) with cidofovir (P < .005). Major virological responses to brincidofovir were also apparent in 9 of 11 cidofovir-unresponsive episodes (Figure 2A-B).
Virological responses to cidofovir and brincidofovir and relationship between complete responses and lymphocyte reconstitution in the 2 groups. (A-B) Shows kinetics of adenoviremia after treatment with cidofovir and brincidofovir, respectively. Viral load (log10 copies/mL) are plotted on the y-axis and days after HCT are plotted on the x-axis. Cidofovir mediated major virological responses (2-log reduction in 2 weeks) in 2 of 23 episodes. In contrast, 13 of 18 episodes demonstrated major virological responses with brincidofovir (P < .0001). The majority of responses to brincidofovir were major. Minor responses (1-log reduction in 2 weeks) were observed only in 2 episodes. Brincidofovir mediated CR in 13 patients (80%) and cidofovir mediated CR in 8 patients (35%) (P < .01). (C) Compares the lymphocyte count at the resolution of viremia following treatment with brincidofovir (n = 15) and cidofovir (n = 8). The median lymphocyte count at the resoluton of viremia after brincidofovir vs cidofovir was 320/μL vs 910/μL, respectively. The resolution of viremia occurred in the brincidofovir group despite significant lymphopenia of >300/μL. In contrast, resolution of viremia occurred in the cidofovir group when lymphocyte count was >300/μL (P < .05). ADV, adenovirus; BCV, brincidofovir; CDV, cidofovir, CR, complete response.
Virological responses to cidofovir and brincidofovir and relationship between complete responses and lymphocyte reconstitution in the 2 groups. (A-B) Shows kinetics of adenoviremia after treatment with cidofovir and brincidofovir, respectively. Viral load (log10 copies/mL) are plotted on the y-axis and days after HCT are plotted on the x-axis. Cidofovir mediated major virological responses (2-log reduction in 2 weeks) in 2 of 23 episodes. In contrast, 13 of 18 episodes demonstrated major virological responses with brincidofovir (P < .0001). The majority of responses to brincidofovir were major. Minor responses (1-log reduction in 2 weeks) were observed only in 2 episodes. Brincidofovir mediated CR in 13 patients (80%) and cidofovir mediated CR in 8 patients (35%) (P < .01). (C) Compares the lymphocyte count at the resolution of viremia following treatment with brincidofovir (n = 15) and cidofovir (n = 8). The median lymphocyte count at the resoluton of viremia after brincidofovir vs cidofovir was 320/μL vs 910/μL, respectively. The resolution of viremia occurred in the brincidofovir group despite significant lymphopenia of >300/μL. In contrast, resolution of viremia occurred in the cidofovir group when lymphocyte count was >300/μL (P < .05). ADV, adenovirus; BCV, brincidofovir; CDV, cidofovir, CR, complete response.
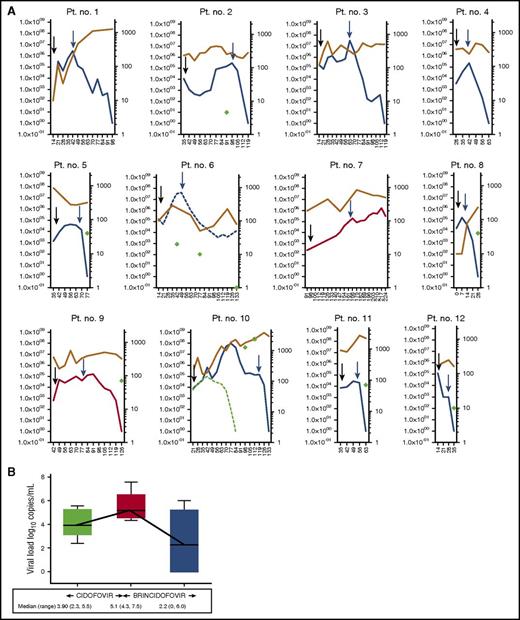
Kinetics of adenoviremia in 12 patients treated with cidofovir followed by brincidofovir. (A) Kinetics of adenoviremia (plotted on left y-axis) and corresponding lymphocyte count (plotted on right y-axis) in 12 episodes treated with cidofovir followed by brincidofovir. Days after HCT are plotted on x-axis. Black arrows indicate the start of cidofovir and blue arrows indicate the start of brincidofovir. Major (blue line) and minor (broken blue line) responses were observed in 9 of 11 episodes unresponsive to cidofovir. No response in 2 weeks is shown as red line. The circulating lymphocyte count is shown as brown line and CD4+ T-cell count (where available) is shown as green diamonds. The median circulating lymphocyte count at CR was 300/μL (160 to 3000). In patient number 10, coexistent CMV viremia (broken green line) resulted in T-cell expansion and resolution of CMV viremia. However, adenoviremia and gut adenoviral disease continued despite CD4+ T-cell reconstitution. Adenovirus-specific T-cell response was absent despite CD4+ T-cell expansion (shown as green circle). In the remaining 7 patients who had lymphocyte subsets measured, CD4+ T cells were <100/μL. (B) Change in adenovirus load between cidofovir and brincidofovir in patients unresponsive to cidofovir (n = 11). The median change in log10 viral load on cidofovir treatment was +1.2 (range, 0.3 to 2.3). In contrast, median change in log10 viral load after 2 weeks of brincidofovir treatment was −2.9 (range, −5.1 to 0.6; P < .005). The solid lines represent median and IQR, and whiskers represent minimum and maximum values. Pt, patient.
Kinetics of adenoviremia in 12 patients treated with cidofovir followed by brincidofovir. (A) Kinetics of adenoviremia (plotted on left y-axis) and corresponding lymphocyte count (plotted on right y-axis) in 12 episodes treated with cidofovir followed by brincidofovir. Days after HCT are plotted on x-axis. Black arrows indicate the start of cidofovir and blue arrows indicate the start of brincidofovir. Major (blue line) and minor (broken blue line) responses were observed in 9 of 11 episodes unresponsive to cidofovir. No response in 2 weeks is shown as red line. The circulating lymphocyte count is shown as brown line and CD4+ T-cell count (where available) is shown as green diamonds. The median circulating lymphocyte count at CR was 300/μL (160 to 3000). In patient number 10, coexistent CMV viremia (broken green line) resulted in T-cell expansion and resolution of CMV viremia. However, adenoviremia and gut adenoviral disease continued despite CD4+ T-cell reconstitution. Adenovirus-specific T-cell response was absent despite CD4+ T-cell expansion (shown as green circle). In the remaining 7 patients who had lymphocyte subsets measured, CD4+ T cells were <100/μL. (B) Change in adenovirus load between cidofovir and brincidofovir in patients unresponsive to cidofovir (n = 11). The median change in log10 viral load on cidofovir treatment was +1.2 (range, 0.3 to 2.3). In contrast, median change in log10 viral load after 2 weeks of brincidofovir treatment was −2.9 (range, −5.1 to 0.6; P < .005). The solid lines represent median and IQR, and whiskers represent minimum and maximum values. Pt, patient.
Virological responses following the use of cidofovir have been described to be mediated by immune recovery.8,9 Therefore, to study the confounding effect of immune reconstitution on virological responses, we compared the circulating lymphocytes at the initiation of brincidofovir and at 2-log reduction in viral load. The median lymphocyte count prior to brincidofovir was 250/μL (range, 0 to 4100) compared with 320/μL (range, 0 to 4300) on 2-log reduction in the viral load (P = NS). Ten of the 15 brincidofovir responses occurred despite ≥1 mg/kg methylprednisolone (supplemental Figure 4A-B). In addition, at complete resolution of viremia, the median lymphocyte count in the brincidofovir group (n = 15) was 320/μL (range, 0 to 3000) compared with 910/μL (range, 380 to 2000) in the cidofovir group (n = 8) (Figure 1C). Thus, the majority of patients were responsive to brincidofovir despite having an absolute lymphocyte count ≤300/μL, which has previously been described as associated with mortality from adenoviral infection post-stem cell transplant11,13 and despite concomitant steroid treatment of GVHD (P < .05).
We determined the relevance of virological responses and the clinical risk of disseminated viral infection. In this study cohort, 2 patients treated with cidofovir died of disseminated adenoviral infection. The AUC of viral load was significantly higher in these patients with virus-related mortality than those who died because of other causes (P < .02) and those who did not suffer mortality (P < .0001; supplemental Figure 2). It is noteworthy that the AUC of viral load in the 4 patients treated with brincidofovir as a first-line drug was significantly lower than the AUC of virus-related mortality (P < .0004). This is an important observation and requires further studies to demonstrate the role of brincidofovir in reducing the risk of adenovirus-related morbidity and mortality.
Adenovirus-related complications can also be reduced by adenovirus-specific T-cell therapy using interferon γ-capture technology. This technology allows rapid collection of adenovirus-specific T cells from the donor; however, a significant investment in infrastructure and expertise is required.14,15 Further, in a recent study of adenovirus-specific T helper 1 cell therapy,14 only 4 (15%) of 26 patients were major responders and a quarter of patients were nonresponders, all of whom died. In addition, adenovirus T-cell therapy did not prevent mortality if organs were involved prior to T-cell transfer. In another study of 5 pediatric HCT recipients, only 1 patient achieved major virological response.15 These observations emphasize the importance of an effective preemptive therapy to prevent progression to adenoviral disease.
There were 3 nonresponders in our study, of which 1 patient had stage 4 gut GVHD. These failed responses may be because of differences in gut absorption, and hence drug pharmacokinetic studies may help to understand failed virological responses and, an IV formulation may improve virological responses in patients with gut GVHD.16 One possible reason for failure to respond is acquisition of adenoviral resistance to brincidofovir, although previous trials have shown infrequent occurrence of brincidofovir resistance.17
Brincidofovir was well-tolerated and only 1 patient required interruption of brincidofovir after 4 weeks because of severe abdominal cramps and diarrhea. Brincidofovir-mediated diarrhea was reported as a frequent side-effect in the phase 3 double-blind placebo controlled trial for preventing CMV infection,18 leading to an eightfold increase in the use of steroids in the brincidofovir arm due to presumed diagnosis of gut GVHD. Hence, it is important to distinguish brincidofovir toxicity from gut GVHD and virus-associated diarrhea in post-HCT patients. Brincidofovir was not observed to mediate nephrotoxicity. In contrast, 9 (39%) of 23 patients treated with cidofovir developed mild-to-moderate nephrotoxicity as previously described (supplemental Figure 5).7
In conclusion, we demonstrate that brincidofovir has a good safety profile and excellent antiadenoviral activity, even during the lymphopenic phase of HCT. Therefore, brincidofovir may be a useful preemptive therapy for HCT-related adenoviremia. Despite the limitations of a retrospective study, this is the largest reported study on the use of brincidofovir for adenovirus to date, and reports contemporary experience from multiple centers. Further prospective studies of this agent are now indicated to confirm the antiadenoviral effect of brincidofovir in the absence of virus-specific responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Chimerix for providing brincidofovir through an expanded access program.
Authorship
Contribution: P.H., P.A., P.V., and R.W. designed research; P.H. collated the data and performed statistical analysis; C.O., P.S., H.D., M.S., T.P., K.P., S.H.L., S.L., C.S., A.G., J.S., and K.R. contributed the data; and P.H. and R.W. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the United Kingdom Paediatric Bone Marrow Transplant Group appears in “Appendix.”
Correspondence: Prashant Hiwarkar, Molecular Immunology Unit, Institute of Child Health, London WC1N 3EH, United Kingdom; e-mail: phiwarkar@nhs.net.
Appendix: study group members
The members of the United Kingdom Paediatric Bone Marrow Transplant Group are: A.G., Ajay Vora, Alice Norton, Beki James, Brenda Gibson, C.S., Denise Bonney, Geoff Shenton, Josu de la Fuente, K.R., K.P., Leena Karnik, M.S., Michelle Cummins, Mike Potter, P.V., P.A., P.H., Robert Chiesa, R.W., Roderick Skinner, and S.L.