To the editor:
Shwachman-Diamond syndrome (SDS) is an inherited multisystem disorder. The clinical diagnostic criteria include evidence of bone marrow failure and exocrine pancreatic dysfunction. The diagnosis is supported by having metaphyseal dysplasia, short stature, and social-behavioral concerns.1,2 Most patients have mutations in the SBDS gene.3 SBDS has been implicated in multiple cellular pathways4-8 ; however, its most prominent studied role is the release of eIF6 from the 60S ribosome subunit enabling monosome 80S ribosome formation.4
Approximately 10% to 20% of SDS patients do not have mutations in SBDS.2,3 Herein, we describe 4 patients with a clinical diagnosis of SDS, from 3 unrelated families, who were negative for mutations in SBDS but carried biallelic mutations in DNAJC21. Based on the clinical cases, comparable ribosomal function of DNAJC21 and SBDS, and recently identified association of DNAJC21 mutations with bone marrow failure,9 we propose that mutations in DNAJC21 cause SDS.
The patients were enrolled in the Canadian Inherited Marrow Failure Registry or in a genetic study of Shwachman-Diamond syndrome. Both studies were approved by the Research Ethics Board at the Hospital for Sick Children, with informed consent.
Whole-exome sequencing, genome-wide analysis of copy number variations, Sanger sequencing, immunoblotting, and gene knockdown by shRNA were performed as described.10-12 Detailed information of Methods and Web resources are in supplemental Data, available on the Blood Web site.
Patient 1 of Family 1 was of Afghani ancestry from consanguineous parents. She presented at the age of 2.5 years with failure to thrive and was found to have pancytopenia, high mean corpuscular volume, and increased hemoglobin F (Table 1). The bone marrow was hypocellular (Figure 1A-B). She also had exocrine pancreatic dysfunction with low serum pancreatic enzyme levels (Table 1), markedly increased echogenicity of the pancreas by ultrasound examination, and hypodense pancreas by computed tomography scan, consistent with lipomatosis (Figure 1C-D). The patient was treated with fat-soluble vitamin supplements. Her height was below the third percentile. Skeletal survey showed evidence of metaphyseal dysplasia (Figure 1E), as described in SDS.13
Patients with mutations in DNAJC21 meet clinical criteria for SDS
| . | Patient 1 . | Patient 2 (sibling of Patient 3) . | Patient 3 (sibling of Patient 2) . | Patient 4 . |
|---|---|---|---|---|
| Age at presentation | 2.5 y | After birth | After birth | After birth |
| Age at diagnosis with SDS | 2 y 11 mo | <1 y | 7 mo | 2 y 2 mo |
| Age at last follow-up or death | 14 y | 1.5 y | 7 y 4 mo | 11 y |
| Evidence of bone marrow failure (age at testing) | Moderately low blood counts (from 2.5 y) | Severe bone marrow failure on chronic transfusion since birth, was planned for transplantation | Severe pancytopenia (7 y), underwent bone marrow transplantation | Severe pancytopenia (2 y), underwent bone marrow transplantation |
| Neutrophils (>1.5 × 109/L) | 1.08 (3 y), 3.54 (14 y) | Severely low | 1.3 (8 mo), 0.5 (7 y) | 0.4 (2 y) |
| Hemoglobin (>120 g/L) | 67 (3 y), 118 (14 y) | Severely low | 97 (8 mo), 55 (7 y) | 74 (2 y) |
| Platelets (>150 × 109/L) | 22 (3 y), 118 (14 y) | Severely low | 185 (8 mo), 17 (7 y) | 17 (2 y 2 mo) |
| Lymphocytes (>1.5 × 109/L) | 2.56 (3 y), 2.28 (14 y) | UK | 5.83 (8 mo), 1.82 (7 y) | 3.4 (2 y) |
| Reticulocytes (>40 × 10°/L) | 96 (14 y) | UK | 102 (8 mo), 33 (7 y) | 32.5 (2 y 2 mo) |
| MCV (0.5-3 y: 70-86 fL, 7-14 y: 75-96) | 89 (3 y), 94 (14 y) | UK | 77 (8 mo), 83 (7 y) | 87 (2 y) |
| HgF (%) (after 1 y <1.2%) | 29 (2.5 y) | UK | 33 | 11.7 (2 y 2 mo) |
| Bone marrow cellularity* (%) | 40-50 (2.5 y) | Markedly reduced | Mildly reduced (8 mo), 10-50% (7 y) | 10 (2 y 5 mo) |
| Prominent marrow dysplasia | No | No | No | No |
| Marrow cytogenetics | N | N | 46,XY, der(15)t(1;15)(q12;p11) (8 mo-7 y) | N |
| Evidence of pancreatic dysfunction | ||||
| Chronic diarrhea | No | No | No | No, fecal fat in microscopy |
| Lipase (23-300 μ/L) | 22 (2 y 11 mo) | UK | 15 (8 mo) | <25 (2 y 2 mo) |
| Amylase (20-110 μ/L) | 36 (2 y 11mo) | UK | 37 (8 mo) | <30 (2 y 3 mo) |
| Pancreatic isoamylase (≥17 μ/L)† | 10 (2 y 11 mo) | UK | 2 (8 mo) | NA |
| Trypsinogen (>16.6 µg/L) | 12.7 (2 y 11 mo) | 11.9 (12 mo), 9.2 (13 mo) | 20 (8 mo) | 3.4 (2 y 2 mo) |
| Vitamin A levels (0.7-2.1 mmol/L) | 1.0 (2 y 11 mo) | UK | 1.4 (8 mo) | 1.4 (2 y 2 mo) |
| Vitamin D levels 70-250 nmol/L) | 60 (2 y 11 mo) | UK | 39 (8 mo) | 69 (5 y 10 mo) |
| Vitamin E levels (12-46 µmol/L) | 9.3 (2 y 11 mo) | UK | 5.2 (3 y) | 8.4 (2 y 2 mo) |
| INR (0.9-1.1) | 0.9 (2 y 11 mo) | UK | 0.97 (8 mo) | NA |
| Hyperechogenic pancreas on US | Markedly echogenic | UK | Markedly echogenic | Diffusely echogenic |
| Hypodense pancreas on CT | Hypodense pancreas | UK | Hypodense pancreas | NA |
| Patient was diagnosed with pancreatic insufficiency before death. On autopsy (18 mo) he was found to have atrophy of exocrine pancreas with fatty infiltration (no changes in the endocrine pancreas) | Autopsy (7 y 4 mo): pancreatic acinar atrophy | |||
| Metaphyseal dysplasia | Yes | Anomaly of the wrist bone (no further details) | Yes (distal radius and ulna, distal femur, proximal tibia) | No, but have osteopenia and focal metaphyseal irregularity in right proximal femur |
| Cognitive impairment | Yes | UK | Yes | Delayed gross motor development |
| Stature | <3% | 30% (11 mo) | <5% (7.5 mo) | <3% |
| Liver | No hepatomegaly, no elevated liver enzymes | Mild fatty changes of the liver (on autopsy) | No hepatomegaly/mild elevation of liver enzymes in infancy | No hepatomegaly, mild elevation of the liver enzymes |
| Other medical problems | Retinitis pigmentosa, generalized seizures (6 mo, 10 mo), severe eczema, asthma, recurrent otitis media, subtle minor physical malformation (low anterior hairline, deep-set eyes, mildly cupped ears, down-turned corners of the mouth, a horizontal crease on the chin, broad neck and redundant skin, increased creases in the palms and deep folds bilaterally, no hearing deficits | Died at the age of 18 mo due to Staphylococcus aureus sepsis | Retinal dystrophy without pigmentation, low visual acuity, decrease visual field, persistent eczema, failure to thrive, kyphosis, mild high-foot inversion, abnormal dentition, hypopigmentation macule at right thigh (developed after transplant) | |
| Other tests with normal results | Chromosomal breakage studies, telomere length, next-generation sequencing of a panel of known 72 IBMFS genes, targeted sequencing of SBDS, urine organic acid and plasma amino acids, 4X180K oligonucleotide array (Agilent Tech), Affymetrix 6.0 Array | Chromosomal breakage studies, targeted sequencing of SBDS, RMRP, Affymetrix 6.0 Array | Chromosomal breakage studies | |
| Targeted sequencing of SBDS, RMRP, Affymetrix 6.0 Array | ||||
| Mitochondrial DNA deletion analysis (for Pearson syndrome) |
| . | Patient 1 . | Patient 2 (sibling of Patient 3) . | Patient 3 (sibling of Patient 2) . | Patient 4 . |
|---|---|---|---|---|
| Age at presentation | 2.5 y | After birth | After birth | After birth |
| Age at diagnosis with SDS | 2 y 11 mo | <1 y | 7 mo | 2 y 2 mo |
| Age at last follow-up or death | 14 y | 1.5 y | 7 y 4 mo | 11 y |
| Evidence of bone marrow failure (age at testing) | Moderately low blood counts (from 2.5 y) | Severe bone marrow failure on chronic transfusion since birth, was planned for transplantation | Severe pancytopenia (7 y), underwent bone marrow transplantation | Severe pancytopenia (2 y), underwent bone marrow transplantation |
| Neutrophils (>1.5 × 109/L) | 1.08 (3 y), 3.54 (14 y) | Severely low | 1.3 (8 mo), 0.5 (7 y) | 0.4 (2 y) |
| Hemoglobin (>120 g/L) | 67 (3 y), 118 (14 y) | Severely low | 97 (8 mo), 55 (7 y) | 74 (2 y) |
| Platelets (>150 × 109/L) | 22 (3 y), 118 (14 y) | Severely low | 185 (8 mo), 17 (7 y) | 17 (2 y 2 mo) |
| Lymphocytes (>1.5 × 109/L) | 2.56 (3 y), 2.28 (14 y) | UK | 5.83 (8 mo), 1.82 (7 y) | 3.4 (2 y) |
| Reticulocytes (>40 × 10°/L) | 96 (14 y) | UK | 102 (8 mo), 33 (7 y) | 32.5 (2 y 2 mo) |
| MCV (0.5-3 y: 70-86 fL, 7-14 y: 75-96) | 89 (3 y), 94 (14 y) | UK | 77 (8 mo), 83 (7 y) | 87 (2 y) |
| HgF (%) (after 1 y <1.2%) | 29 (2.5 y) | UK | 33 | 11.7 (2 y 2 mo) |
| Bone marrow cellularity* (%) | 40-50 (2.5 y) | Markedly reduced | Mildly reduced (8 mo), 10-50% (7 y) | 10 (2 y 5 mo) |
| Prominent marrow dysplasia | No | No | No | No |
| Marrow cytogenetics | N | N | 46,XY, der(15)t(1;15)(q12;p11) (8 mo-7 y) | N |
| Evidence of pancreatic dysfunction | ||||
| Chronic diarrhea | No | No | No | No, fecal fat in microscopy |
| Lipase (23-300 μ/L) | 22 (2 y 11 mo) | UK | 15 (8 mo) | <25 (2 y 2 mo) |
| Amylase (20-110 μ/L) | 36 (2 y 11mo) | UK | 37 (8 mo) | <30 (2 y 3 mo) |
| Pancreatic isoamylase (≥17 μ/L)† | 10 (2 y 11 mo) | UK | 2 (8 mo) | NA |
| Trypsinogen (>16.6 µg/L) | 12.7 (2 y 11 mo) | 11.9 (12 mo), 9.2 (13 mo) | 20 (8 mo) | 3.4 (2 y 2 mo) |
| Vitamin A levels (0.7-2.1 mmol/L) | 1.0 (2 y 11 mo) | UK | 1.4 (8 mo) | 1.4 (2 y 2 mo) |
| Vitamin D levels 70-250 nmol/L) | 60 (2 y 11 mo) | UK | 39 (8 mo) | 69 (5 y 10 mo) |
| Vitamin E levels (12-46 µmol/L) | 9.3 (2 y 11 mo) | UK | 5.2 (3 y) | 8.4 (2 y 2 mo) |
| INR (0.9-1.1) | 0.9 (2 y 11 mo) | UK | 0.97 (8 mo) | NA |
| Hyperechogenic pancreas on US | Markedly echogenic | UK | Markedly echogenic | Diffusely echogenic |
| Hypodense pancreas on CT | Hypodense pancreas | UK | Hypodense pancreas | NA |
| Patient was diagnosed with pancreatic insufficiency before death. On autopsy (18 mo) he was found to have atrophy of exocrine pancreas with fatty infiltration (no changes in the endocrine pancreas) | Autopsy (7 y 4 mo): pancreatic acinar atrophy | |||
| Metaphyseal dysplasia | Yes | Anomaly of the wrist bone (no further details) | Yes (distal radius and ulna, distal femur, proximal tibia) | No, but have osteopenia and focal metaphyseal irregularity in right proximal femur |
| Cognitive impairment | Yes | UK | Yes | Delayed gross motor development |
| Stature | <3% | 30% (11 mo) | <5% (7.5 mo) | <3% |
| Liver | No hepatomegaly, no elevated liver enzymes | Mild fatty changes of the liver (on autopsy) | No hepatomegaly/mild elevation of liver enzymes in infancy | No hepatomegaly, mild elevation of the liver enzymes |
| Other medical problems | Retinitis pigmentosa, generalized seizures (6 mo, 10 mo), severe eczema, asthma, recurrent otitis media, subtle minor physical malformation (low anterior hairline, deep-set eyes, mildly cupped ears, down-turned corners of the mouth, a horizontal crease on the chin, broad neck and redundant skin, increased creases in the palms and deep folds bilaterally, no hearing deficits | Died at the age of 18 mo due to Staphylococcus aureus sepsis | Retinal dystrophy without pigmentation, low visual acuity, decrease visual field, persistent eczema, failure to thrive, kyphosis, mild high-foot inversion, abnormal dentition, hypopigmentation macule at right thigh (developed after transplant) | |
| Other tests with normal results | Chromosomal breakage studies, telomere length, next-generation sequencing of a panel of known 72 IBMFS genes, targeted sequencing of SBDS, urine organic acid and plasma amino acids, 4X180K oligonucleotide array (Agilent Tech), Affymetrix 6.0 Array | Chromosomal breakage studies, targeted sequencing of SBDS, RMRP, Affymetrix 6.0 Array | Chromosomal breakage studies | |
| Targeted sequencing of SBDS, RMRP, Affymetrix 6.0 Array | ||||
| Mitochondrial DNA deletion analysis (for Pearson syndrome) |
CT, computed tomography; HgF, hemoglobin F; MCV, mean corpuscular volume (red blood cells); N, normal; UK, unknown; US, ultrasound.
Quantification of cellularity in percentages was not available and commonly used clinical terms to describe bone marrow cellularity were reported to the registry. We refer to “markedly reduced” cellularity in children as <25% and “mildly reduced” cellularity as “50-75%.”
Reference levels are from ∼3 years of age onward.
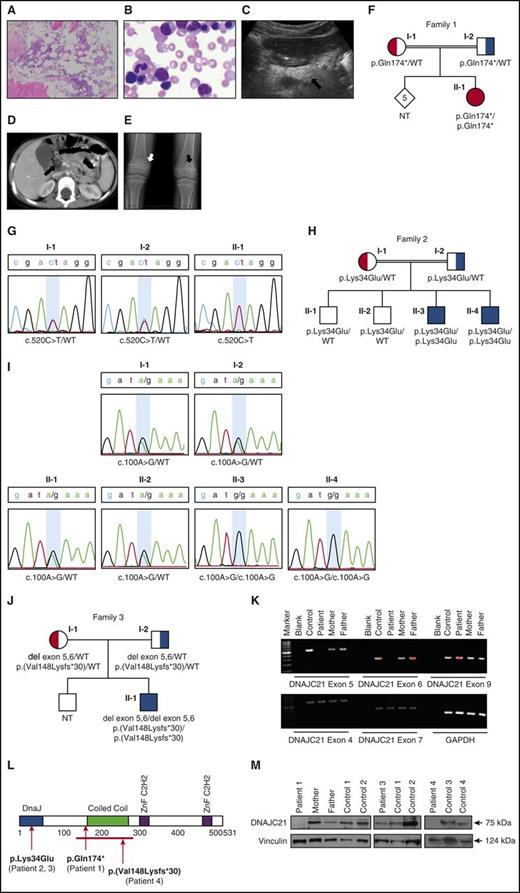
Clinical characteristics of Patient 1. (A) Reduced cellularity is apparent in the marrow biopsy. Hematopoietic activities occupy ∼40% of the space between bones. Surrounding fat tissue is seen. Original magnification ×100. (B) Bone marrow aspirate shows no evidence of dysplasia, cytoplasmic vacuoles, ringed sideroblasts, or excess blasts. Original magnification ×500. (C) Imaging of the pancreas showed hyperechogenicity (arrow) on ultrasonography (imaging of a normal pancreas can be found in supplemental Figure 2D). (D) Computed tomography scan showed hypodense tissue (arrow). The ultrasound and computed tomography images are characteristic features of lipomatosis seen in SDS. (E) Radiograph of the long bones showed irregularity of the metaphyses with areas of sclerosis (black arrow) and lucency (white arrow). Similar images of Patient 3 can be found in supplemental Figure 1. (F) Pedigree of Family 1. (G) Sanger sequencing was done to validate mutations in DNAJC21 (NM_001012339.2) found by whole-exome sequencing. The figure indicates the position of the mutation (c.520) on exon 5. The nucleotide substitution of C by T is seen in homozygous (patient, II-1) and heterozygous (parents, I-1 and I-2) states, consistent with recessive inheritance. The alteration results in premature protein truncation (p.Gln174*). (H) Pedigree of Family 2. (I) Sanger sequencing of portion of exon 2 of DNAJC21 in family members showing segregation of the pathogenic missense variant. (J) Pedigree of Family 3. (K) PCR of genomic DNA from patient 4 showing absence of amplification of exons 5 and 6, and normal amplification of surrounding exons and glyceraldehyde-3-phosphate dehydrogenase control. (L) The DNACJ21 protein with its domains and predicted patients’ mutations are shown. (M) Immunoblots of protein extracts of cells from controls and members of Family 1 (left, peripheral blood T cells), Patient 2 (center, peripheral blood T cells), and Patient 4 (right, marrow fibroblasts) with DNAJC21 and loading control antibodies. Band densitometry was performed with ImageJ software. NT, not tested.
Clinical characteristics of Patient 1. (A) Reduced cellularity is apparent in the marrow biopsy. Hematopoietic activities occupy ∼40% of the space between bones. Surrounding fat tissue is seen. Original magnification ×100. (B) Bone marrow aspirate shows no evidence of dysplasia, cytoplasmic vacuoles, ringed sideroblasts, or excess blasts. Original magnification ×500. (C) Imaging of the pancreas showed hyperechogenicity (arrow) on ultrasonography (imaging of a normal pancreas can be found in supplemental Figure 2D). (D) Computed tomography scan showed hypodense tissue (arrow). The ultrasound and computed tomography images are characteristic features of lipomatosis seen in SDS. (E) Radiograph of the long bones showed irregularity of the metaphyses with areas of sclerosis (black arrow) and lucency (white arrow). Similar images of Patient 3 can be found in supplemental Figure 1. (F) Pedigree of Family 1. (G) Sanger sequencing was done to validate mutations in DNAJC21 (NM_001012339.2) found by whole-exome sequencing. The figure indicates the position of the mutation (c.520) on exon 5. The nucleotide substitution of C by T is seen in homozygous (patient, II-1) and heterozygous (parents, I-1 and I-2) states, consistent with recessive inheritance. The alteration results in premature protein truncation (p.Gln174*). (H) Pedigree of Family 2. (I) Sanger sequencing of portion of exon 2 of DNAJC21 in family members showing segregation of the pathogenic missense variant. (J) Pedigree of Family 3. (K) PCR of genomic DNA from patient 4 showing absence of amplification of exons 5 and 6, and normal amplification of surrounding exons and glyceraldehyde-3-phosphate dehydrogenase control. (L) The DNACJ21 protein with its domains and predicted patients’ mutations are shown. (M) Immunoblots of protein extracts of cells from controls and members of Family 1 (left, peripheral blood T cells), Patient 2 (center, peripheral blood T cells), and Patient 4 (right, marrow fibroblasts) with DNAJC21 and loading control antibodies. Band densitometry was performed with ImageJ software. NT, not tested.
The patient also had gross and fine motor developmental delay and bilateral retinal dystrophy (supplemental Figure 1A-D).
The second family included 2 affected children to consanguineous parents of First Nations (Canada) ancestry. The first sibling (Patient 2) was diagnosed after birth with progressive bone marrow failure (Table 1) and died of Staphylococcus aureus sepsis at the age of 18 months. He was diagnosed with pancreatic insufficiency and SDS based on low serum trypsinogen levels before his death. Autopsy demonstrated atrophic exocrine pancreas with fatty infiltration and preservation of the endocrine tissue.
The second sibling (Patient 3) had pancytopenia with severe anemia after birth, but later had stable moderate pancytopenia. Bone marrow testing at 8 months of age showed hypocellularity (supplemental Figure 2A-B), with a cytogenetic abnormality 46,XY, der(15)t(1;15)(q12;p11). Peripheral blood karyotype was normal. At 7 years of age, he developed severe pancytopenia and underwent bone marrow transplantation, but died 2.5 months post-transplant of Epstein-Barr virus–associated lymphoproliferative disorder. The patient also had feeding difficulties, low serum pancreatic enzyme levels (Table 1), and a small hyperechogenic pancreas (supplemental Figure 2C-D). He was treated with fat-soluble vitamin supplements. Metaphyseal dysplasia in multiple joints (supplemental Figure 2E-F and Table 1) and mild flaring of anterior rib ends as described in SDS13 were found at presentation.
The third family included one affected patient (Patient 4) to parents of Indian descent. The patient developed severe aplastic anemia at 2 years of age (Table 1) and underwent successful bone marrow transplantation. He had evidence of exocrine pancreatic dysfunction, metaphyseal dysplasia, short stature, and developmental delay, and was diagnosed with SDS. He developed retinal dysplasia after transplant.
Relevant clinical diagnostic tests done on the patients are described in Table 1. Given the typical hematologic, pancreatic, and skeletal findings, all were diagnosed with SDS, according to published international consensus guidelines.1
We performed whole-exome sequencing on peripheral blood from the patient and parents of Family 1 as described.12 Pedigree is shown in Figure 1F. DNAJC21 was considered to be the candidate gene because of a homozygous stop codon variant (c.520C>T, p.Gln174*) that was not described in studied populations (supplemental Table 1), and the currently understood gene function in ribosome biogenesis that resembles SBDS. Homozygosity in the patient and heterozygosity in the parents were validated by Sanger sequencing (Figure 1G).
Based on identification of an extended run of homozygosity including DNAJC21 by SNP6.0 array in Patient 3 (supplemental Figure 3) and the parents’ consanguinity (Figure 1H), we also considered DNAJC21 in Family 2. Sanger sequencing of all exons revealed a previously unreported homozygous mutation c.100A>G that results in substitution of a highly conserved amino acid p.Lys34Glu in the J-domain (Figure 1I). This variant is predicted to be damaging and disease-causing by multiple protein prediction software programs (supplemental Table 1). Using the protein structure prediction software CFSSPS, the mutation was predicted to lengthen an α-helical segment and abolish a turn within the DnaJ domain (supplemental Figure 4). Based on the available NMR structure of the J domain of murine polyoma T antigen (1FAF) (http://www.rcsb.org/pdb), residue K34 is exposed on the solvent side of the protein; thus, the mutation is likely to reverse the surface charge and may not perturb the protein fold.
Exome sequencing of Family 3 (Figure 1J) did not reveal nucleotide-level mutations; however, careful examination of Patient 4’s DNAJC21 sequence revealed only few reads from exons 5 and 6 in contrast to surrounding exons (supplemental Figure 5A). Parents had borderline read numbers in these regions. Analysis of the exome data by NextGene 2.4.2 indicated a deletion of these exons with high confidence (supplemental Figure 5B-C). Polymerase chain reaction (PCR) of genomic DNA showed no amplification of exons 5 and 6, consistent with their deletion (Figure 1K). This is predicted to cause splicing that merges exon 4 with exon 7, frameshift, and early protein truncation p.(Val148Lysfs*30). Reverse transcription of T-cell RNA, followed by PCR of the cDNA fragment that includes exon 4-7 showed heterozygosity for a long (normal) and a short (mutant) allele in the parents and homozygosity for the short allele in the child (supplemental Figure 5D). Sequencing confirmed the absence of exon 5-6 in the short fragment (supplemental Figure 5E).
DNAJC21 encodes a protein14 with a common isoform of 531 amino acids. It contains a highly conserved amino-terminal DnaJ-domain,15 a centrally positioned coiled coil region, and a carboxyl segment with two C2H2-type zinc fingers that flank 168 amino acids that are rich in charged residues.16 The protein structure and location of the mutations are depicted in Figure 1L. Immunoblotting revealed markedly reduced protein levels in cells from Patient 1 (homozygous nonsense mutation) and Patient 4 (biallelic exon 5-6 deletion) compared with noncarrier controls, and ∼40% reduction in Patient 3 (homozygous missense mutation) (Figure 1M). The specificity of the antibody was demonstrated by shRNA-mediated DNAJC21 knockdown in HEK-293T cells (supplemental Figure 6). The patients did not have prominent reduction in the SBDS protein (supplemental Figure 7), as typically seen in SDS patients with mutations in SBDS.
DNAJC21 is ubiquitously expressed. Its homolog in Saccharomyces cerevisiae, Jjj1, is required for ribosome biogenesis through the DnaJ-domain. Both SBDS and DNAJC21 homologs are involved in the release of maturation/auxiliary factors from the pre-60S ribosomal subunit, Tif6 by Sdo1 and Arx1/Alb1 by Jjj1. Yeast strains deleted for these homologs lead to accumulation of their target factors in the cytosol,17,18 grow poorly at low temperatures,17,19 accumulate half-mer ribosomes,4,20 and have reduced levels of mature ribosomes16,17 —all hallmarks of dysfunctional 60S ribosomal subunit biogenesis. Efficient release of Tif6 from 60S is also dependent on the release of Arx121 ; therefore, Jjj1 deficiency may also affect the release of Tif6. This could be an additional reason for the phenotypic similarity in patients with mutation of DNAJC21 or SBDS.
A recent paper showed that DNAJC21 is associated with bone marrow failure.9 Unfortunately, the presented clinical pictures were limited, and no pancreatic data were indicated, so it remains unclear whether an SDS diagnosis had been considered. Exocrine pancreatic dysfunction is frequently difficult to diagnose, and status can change with age.22 Many challenges in classification of inherited bone marrow failure syndromes have been noted,23,24 but improvements are anticipated as new genes are identified. Of note, an additional 3 SDS patients in our registry had no mutations in SBDS and DNAJC21 by whole-exome sequencing and SNP6.0 array. Thus, it is likely that additional SDS genes remain to be identified.
A number of unique clinical features have been described in individual patients with SDS.25-28 The retinitis pigmentosa in Patient 1 is intriguing, but is possibly caused by a novel homozygous indel/frameshift variant in C2orf7129 (supplemental Figure 1E), which the patient carried in addition to DNAJC21. However, because Patient 4 also had retinal dystrophy (albeit post-transplant) and retinal dystrophy was also observed by Tummala and colleagues,9 an association between DNAJC21 and retinal phenotype is possible.
In summary, our data indicate that mutations in DNAJC21 can lead to SDS. The involvement of DNAJC21 in ribosome functions that parallel those of SBDS highlights the need to better understand how ribosome failure contributes to SDS and related bone marrow failure phenotypes.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This study was supported by grants from the Canadian Institutes of Health Research (286737 [Y.D.] and 102506 [J.M.R.]), the Nicola’s Kids Triathlon (Y.D.), and The Mira Godard Research Fund (E.H.).
Contribution: S.D. and A.M. performed research, analyzed data, and wrote the paper; H.L., S.L., B.Z., and J.Z. performed research; J.M.R., E.H., A.V., M.C., and R.M.-L. contributed vital data and wrote the manuscript; S.W.S. and C.S.T. assisted in developing analytic tools and supervising analysis; L.J. and P.R.D. contributed vital data; and Y.D. designed research, oversaw the project, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yigal Dror, Division of Haematology Oncology, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8, Canada; e-mail: yigal.dror@sickkids.ca.
References
Author notes
S.D. and A.M. contributed equally to the manuscript and are joint first authors.