Key Points
First in human trial of Triplex vaccine shows safety and expansion of durable CMV-specific T cells with potential for viremia control.
Triplex is immunogenic in both CMV-seronegative and -seropositive healthy adults with or without previous smallpox vaccination.
Abstract
Attenuated poxvirus modified vaccinia Ankara (MVA) is a useful viral-based vaccine for clinical investigation, because of its excellent safety profile and property of inducing potent immune responses against recombinant (r) antigens. We developed Triplex by constructing an rMVA encoding 3 immunodominant cytomegalovirus (CMV) antigens, which stimulates a host antiviral response: UL83 (pp65), UL123 (IE1-exon4), and UL122 (IE2-exon5). We completed the first clinical evaluation of the Triplex vaccine in 24 healthy adults, with or without immunity to CMV and vaccinia virus (previous DryVax smallpox vaccination). Three escalating dose levels (DL) were administered IM in 8 subjects/DL, with an identical booster injection 28 days later and 1-year follow-up. Vaccinations at all DL were safe with no dose-limiting toxicities. No vaccine-related serious adverse events were documented. Local and systemic reactogenicity was transient and self-limiting. Robust, functional, and durable Triplex-driven expansions of CMV-specific T cells were detected by measuring T-cell surface levels of 4-1BB (CD137), binding to CMV-specific HLA multimers, and interferon-γ production. Marked and durable CMV-specific T-cell responses were also detected in Triplex-vaccinated CMV-seronegatives, and in DryVax-vaccinated subjects. Long-lived memory effector phenotype, associated with viral control during CMV primary infection, was predominantly found on the membrane of CMV-specific and functional T cells, whereas off-target vaccine responses activating memory T cells from the related herpesvirus Epstein-Barr virus remained undetectable. Combined safety and immunogenicity results of MVA in allogeneic hematopoietic stem cell transplant (HCT) recipients and Triplex in healthy adults motivated the initiation of a placebo-controlled multicenter trial of Triplex in HCT patients. This trial was registered at www.clinicaltrials.gov as #NCT02506933.
Introduction
Cytomegalovirus (CMV) viremia is associated with an increased risk of mortality in the first year after allogeneic hematopoietic stem cell transplant (HCT).1 Despite the implementation of sophisticated strategies for prophylaxis, monitoring, and preemptive treatment, CMV reactivation still occurs in HCT patients.2,3
In HCT recipients, protection from CMV viremia is critically dependent upon the reconstitution and expansion of CMV-specific T-cell subsets, such as pp65 tegument (UL83) and the immediate early 1 (IE1, UL123) T cells.4-8 Until recently, eliciting an immune response by vaccinating the HCT recipient early posttransplant was marginally successful.9 However, CMVPepVax, a peptide vaccine combined with the Pfizer adjuvant PF03512676, provided proof of concept that HCT recipients are able to mount vaccine-driven CMV-specific T-cell response within 6 weeks post-HCT, when they are at the highest risk for CMV reactivation.10-12 CMVPepVax includes an HLA A*0201 restricted CD8 T-cell peptide epitope of the pp65 protein (pp65495–503); thus, its use is restricted to patients who are HLA A*0201 positive.
To broaden the proportion of HCT recipients who could benefit from a therapeutic CMV vaccine, we have developed a Modified vaccinia Ankara (MVA)–based vaccine (Triplex) encoding 3 full-length CMV antigens: pp65, IE1-exon4, and IE2-exon5 (UL122). These antigens are highly recognized in the majority of CMV-seropositive healthy subjects and transplant patients, and their role in protective immunity has been described.4-8,13-16 The concept is to use the capacity of infection and the immunological properties of the live MVA vector to elicit an immune response against the heterologous protein being presented. The attractiveness of MVA for clinical use stems from its previous safety record as a smallpox vaccine in the young and/or elderly,17-21 and as a therapeutic vaccine in both cancer22-25 and immune-suppressed HIV-AIDS patients.26-28 Critical to the clinical development of Triplex was a trial of wild-type nonrecombinant MVA conducted in HCT recipients, which showed safety and immunogenicity.29
We first established Triplex preclinical safety and immunogenicity using HLA transgenic mouse models and human peripheral blood mononuclear cells (PBMC) from CMV-seropositive healthy volunteers and HCT recipients.13 Subsequently, we developed indices of genetic stability, antigen expression, and immunogenicity, which provided a rationale for manufacturing of a clinical grade lot of Triplex.30
The phase 1 trial described in this report was designed to evaluate safety and immunogenicity of Triplex in healthy adults. The rationale was based on a proposed trial of transplant donors who are immunocompetent and equivalent immunologically to healthy adults. We explored tolerability of increasing dose levels (DLs) of the vaccine and assessed whether Triplex stimulated T-cell responses directed toward pp65, IE1-exon4, and IE2-exon5 in CMV-seropositive and -seronegative subjects as well as those who had received smallpox vaccination.
Methods
Study population
This study was approved by the City of Hope Comprehensive Cancer Center (COH, Duarte, CA) institutional review board and was conducted in accordance with Good Clinical Practices and US Food and Drug Administration regulations. Healthy male and female volunteers, ≥18 and ≤60 years old, were recruited among COH employees and were enrolled in the study after signing written informed consent. (Exclusion criteria are available in the supplemental data, available on the Blood Web site.)
Vaccine construct
In collaboration with the National Cancer Institute's Experimental Therapeutics program, we developed Triplex. It was constructed using an MVA24 and 2 plasmid shuttle vectors: mH5-pp65-pLW51 expressing pp65 and mH5-IEfusion-pZWIIA expressing the fusion of IE1-exon4 and IE2-exon5 (Figure 1A; supplemental data).30
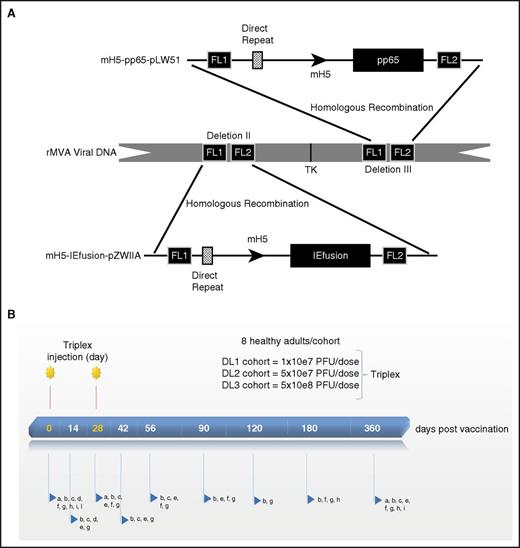
Triplex vaccine construct and vaccination regimen. (A) Vaccine characteristic: schematic representation of Triplex vaccine. Direct repeats have been previously described.34 FL1 and FL2 are flanking (FL) DNA of deletions II and III. Plasmid mH5-pp65-pLW51 and mH5-IEfusion-pZWIIA structures and the modified H5 promoter have been detailed elsewhere.13,34 TK, thymidine kinase gene of MVA.34 Arrows show direction of transcription. (B) Vaccination regimen: 2 injections of Triplex vaccine were administered at each DL as indicated. Postvaccination follow-up days are shown on the bar. Letters pointed by the blue arrows detail laboratory and immune evaluations, clinical and AE assessment. In detail, a, urine pregnancy test (female subjects); b, metabolic and hematologic panels; c, physical examination; d, electrocardiogram and troponin test; e, AE monitoring; f, MVA vector persistence measurements; g, immunological assays; h, CMV serology; I, HIV, hepatitis B and C tests; l, HLA typing at A and B loci. For b, f, g, h, i, and l, drawing blood was required.
Triplex vaccine construct and vaccination regimen. (A) Vaccine characteristic: schematic representation of Triplex vaccine. Direct repeats have been previously described.34 FL1 and FL2 are flanking (FL) DNA of deletions II and III. Plasmid mH5-pp65-pLW51 and mH5-IEfusion-pZWIIA structures and the modified H5 promoter have been detailed elsewhere.13,34 TK, thymidine kinase gene of MVA.34 Arrows show direction of transcription. (B) Vaccination regimen: 2 injections of Triplex vaccine were administered at each DL as indicated. Postvaccination follow-up days are shown on the bar. Letters pointed by the blue arrows detail laboratory and immune evaluations, clinical and AE assessment. In detail, a, urine pregnancy test (female subjects); b, metabolic and hematologic panels; c, physical examination; d, electrocardiogram and troponin test; e, AE monitoring; f, MVA vector persistence measurements; g, immunological assays; h, CMV serology; I, HIV, hepatitis B and C tests; l, HLA typing at A and B loci. For b, f, g, h, i, and l, drawing blood was required.
Study design
This study was an open-label, single-arm, dose-escalating, phase 1 clinical trial to assess safety and immunogenicity of Triplex vaccine, in 24 sequentially enrolled healthy adults. Three escalating DLs of the vaccine were evaluated: DL1 was at the level of 10×e7 plaque forming units (PFU) of Triplex; DL2 of 5×10e7 PFU; DL3 of 5×10e8 PFU. Each DL was assessed in a cohort of 8 subjects, who received the vaccine in a volume of 1 mL by intramuscular (IM) route in the upper arm, and an identical booster injection 28 days later (Figure 1B).12 CMV serostatus was assessed for all participants on days 0, 180, and 360 by using Virgo Cytomegalovirus/CMV IgG IFA test kit (Hemagen Diagnostics, Inc, Columbia, MD).
Safety evaluation
At each visit, adverse events (AE; Table 1) were monitored; laboratory tests, including complete blood count with leukocyte differential, and symptom-directed clinical evaluations were performed (Figure 1B). MVA DNAemia persistence was monitored in all vaccinated individuals for up to 1 year.31,32 Real-time polymer chain reaction was used with primers targeting the MVA thymidine kinase gene (Figure 1A).30 The assay was performed in triplicate (0.5 μg cellular DNA/well) with 1 additional sample spiked with 51 copies of plasmid DNA with the identical CMV antigen cassette as contained in Triplex. The assay is sensitive to 2000 genomic units/mL (=20 copies MVA DNA/µg cellular DNA).
AEs to Triplex in all vaccinated subjects
| . | DL1 . | DL2 . | DL3 . | . | |||
|---|---|---|---|---|---|---|---|
| AE* . | 1&2 . | 3 . | 1&2 . | 3 . | 1&2 . | 3 . | % total . |
| Local | |||||||
| Erythema | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Induration | 0 | 0 | 1 | 0 | 1 | 0 | 8 |
| Paresthesia | 0 | 0 | 0 | 0 | 1 | 0 | 4 |
| Systemic | |||||||
| Fatigue | 0 | 0 | 1 | 0 | 8 | 0 | 38 |
| Hyperbilirubinemia | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Myalgia | 2 | 0 | 1 | 0 | 7 | 0 | 42 |
| Hypertension | 0 | 0 | 0 | 0 | 1 | 0 | 4 |
| Nausea | 0 | 0 | 0 | 0 | 2 | 0 | 8 |
| Cough | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Headache | 1 | 0 | 2 | 0 | 5 | 0 | 33 |
| . | DL1 . | DL2 . | DL3 . | . | |||
|---|---|---|---|---|---|---|---|
| AE* . | 1&2 . | 3 . | 1&2 . | 3 . | 1&2 . | 3 . | % total . |
| Local | |||||||
| Erythema | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Induration | 0 | 0 | 1 | 0 | 1 | 0 | 8 |
| Paresthesia | 0 | 0 | 0 | 0 | 1 | 0 | 4 |
| Systemic | |||||||
| Fatigue | 0 | 0 | 1 | 0 | 8 | 0 | 38 |
| Hyperbilirubinemia | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Myalgia | 2 | 0 | 1 | 0 | 7 | 0 | 42 |
| Hypertension | 0 | 0 | 0 | 0 | 1 | 0 | 4 |
| Nausea | 0 | 0 | 0 | 0 | 2 | 0 | 8 |
| Cough | 1 | 0 | 0 | 0 | 0 | 0 | 4 |
| Headache | 1 | 0 | 2 | 0 | 5 | 0 | 33 |
AEs were listed accordingly to the HIV Vaccine Trials Network/Division of AIDS intensity grading scale as: grade 1 = mild; grade 2 = moderate; grade 3 = severe; grade 4 = life-threatening. The table shows the number of volunteers who experienced grade 1 & 2, or 3 local or systemic AEs (listed in the left column, according to the HTVN intensity grading scale) for each vaccine DL (as specified in Table 2) cohort (N = 8) indicated in the boxes. No toxicity grade 4 AE was reported. Last column shows the percentages of the total post-vaccination–related AEs. AEs that were considered unrelated or unlikely to be related to the investigational agent are not shown.
CMV-specific and MVA vector–specific immune analysis
We monitored CMV-specific and MVA vector-specific immune responses in PBMC by longitudinally measuring the T-cell levels of 4-1BB (CD137), binding to HLA multimers, interferon-γ (IFN-γ) production, memory phenotype, and vaccinia virus neutralization assays (see supplemental data for technique details).
Statistical analysis
Longitudinal measurements were analyzed by fitting a piecewise-linear model, allowing for a rise to day 42, and a decline afterward. Piecewise linear models are presented for drawing valid, but approximate inferences about rise and decline of mean concentrations in the context of expected substantial variability in CMV-specific T-cell baseline concentrations among participants.15,33 Responses were modeled on a square-root scale. Models were fit using generalized estimating equations (GEE, using the “gee” package in R). Additional terms were fitted in GEE framework to evaluate variation due to dose, CMV serostatus, and previous vaccinia exposure. Triplex responders were defined as those who showed a persistently higher than baseline concentration of pp65-, IE1-exon4, or IE2-exon5-specific CD137+ CD8+ or CD4+ T cells through day 360. Wilcoxon signed rank test was used to address statistical significance of changes at specific days postvaccination. Pearson (“r”) correlation coefficient was used for correlation tests. Analysis of variance was used to obtain broad tests of variation among subjects and phenotype changes postvaccination.
Results
Subject characteristics
COH employees (N = 49) were consecutively enrolled and screened, and 24 were eligible for study participation. All eligible participants received the 2-injection vaccine regimen. None withdrew from the study; 1 subject was lost to follow-up after 180 days due to relocation. Sixteen participants were women (67%), and 79% of individuals were white (4 of Hispanic ethnicity). Subjects ranged in age from 22 to 51 years, with a median age of 28 years (Table 2). Eighteen subjects (75%) were born after 1972 and were presumed to be vaccinia virus naive (did not receive DryVax vaccine). Among participants born when vaccination against smallpox was compulsory (N = 6, Table 2), 5 had a smallpox vaccination scar, although for 1 (unique patient number [UPN] 17), the scar was invisible. Three persons were CMV seronegative at study initiation,33 2 of them (in DL2 and DL3) seroconverted after 180-day blood draw and tested CMV-seropositive on the last study visit (day 360). CMV-seropositivity rate in our cohort was 87.5% (confidence interval 0.68-0.97) at study start, which is a greater frequency than found in almost all locales in the United States. However, the confidence interval associated with the rate found in our trial encompasses the average for Los Angeles County (LAC),34 so it is consistent with the local population. Furthermore, 25% of our study subjects were Hispanic, a group with high CMV positivity both nationally and within LAC, and older (range 23-53 years) than the National Center for Health Statistics survey for LAC (range 6-49 years). It has been reported that CMV seropositivity increases with age.34 Thus, the rates of CMV seropositivity that we measured are expected based on both ethnicity and age of our LAC study population. The first study subject was vaccinated on January 2015, and the last subject received the final 1-year follow-up visit in March 2016. This trial is closed to accrual, and the final analysis is presented in this report.
Demographic characteristics of the vaccinated subjects
| . | DL1* . | DL2† . | DL3‡ . |
|---|---|---|---|
| Number of subjects | 8 | 8 | 8 |
| Median age (range) | 32 (26-51) | 29.5 (25-45) | 37 (24-53) |
| Birth before 1972§ | 2 | 1 | 3 |
| CMV-serology‖ | |||
| Positive | 8 | 6 | 7 |
| Negative | 0 | 2¶ | 1# |
| Sex | |||
| Female | 4 | 5 | 8 |
| Male | 4 | 3 | 0 |
| Race | |||
| Asian/Pacific Islander | 1 | 1 | 1 |
| White | 6 | 7 | 6 |
| Other (multiracial) | 1 | 0 | 1 |
| . | DL1* . | DL2† . | DL3‡ . |
|---|---|---|---|
| Number of subjects | 8 | 8 | 8 |
| Median age (range) | 32 (26-51) | 29.5 (25-45) | 37 (24-53) |
| Birth before 1972§ | 2 | 1 | 3 |
| CMV-serology‖ | |||
| Positive | 8 | 6 | 7 |
| Negative | 0 | 2¶ | 1# |
| Sex | |||
| Female | 4 | 5 | 8 |
| Male | 4 | 3 | 0 |
| Race | |||
| Asian/Pacific Islander | 1 | 1 | 1 |
| White | 6 | 7 | 6 |
| Other (multiracial) | 1 | 0 | 1 |
DL1 = 10×e7 PFU of Triplex.
DL2 = 5×10e7 PFU.
DL3 = 5×10e8 PFU.
Subjects received live vaccinia virus against smallpox.
Assessed by using Virgo Cytomegalovirus/CMV IgG IFA test kit (Hemagen Diagnostics, Inc).
One subject tested CMV-seropositive on day 360.
CMV-seropositive on day 360.
Safety and tolerability
Triplex vaccine was well tolerated in most subjects at all DLs (Table 1). A single grade 3 injection site AE (erythema), which resolved in 1 day without treatment, was reported in a single DL3 subject (UPN 17), who was born in 1970 and presumably received smallpox vaccination. Three (12.5%) mild to moderate cutaneous reactions were recorded. The most common systemic reaction consisted of mild to moderate flulike symptoms and was experienced by all (fatigue) or most DL3 subjects, and by few (1-2) subjects from both DL1 and DL2 cohorts. All systemic reactogenic events were transient and self-limited and resolved without sequelae. No serious AEs were attributable to vaccination. Because of previous findings of myopericarditis in recipients of live vaccinia virus (DryVax vaccine),35 subjects were examined closely for possible cardiac side effects related to immunization: no cardiac AEs and no change in electrocardiogram and cardiac troponin levels postvaccination (Figure 1B) were recorded. MVA viral DNA was undetectable at all tested time points (Figure 1B) in both DL1 and DL2 cohorts. In the DL3 cohort, in which subjects received a total of 10.4×10e8 genomic units of MVA viral DNA, minimal levels of MVA viral DNA were detected on day 90 (48 and 57 copies of MVA DNA/µg cellular DNA for UPN 20 and 22, respectively), and day 360 (113 copies/µg cellular DNA for UPN 20).
CMV-specific T cells in research subjects
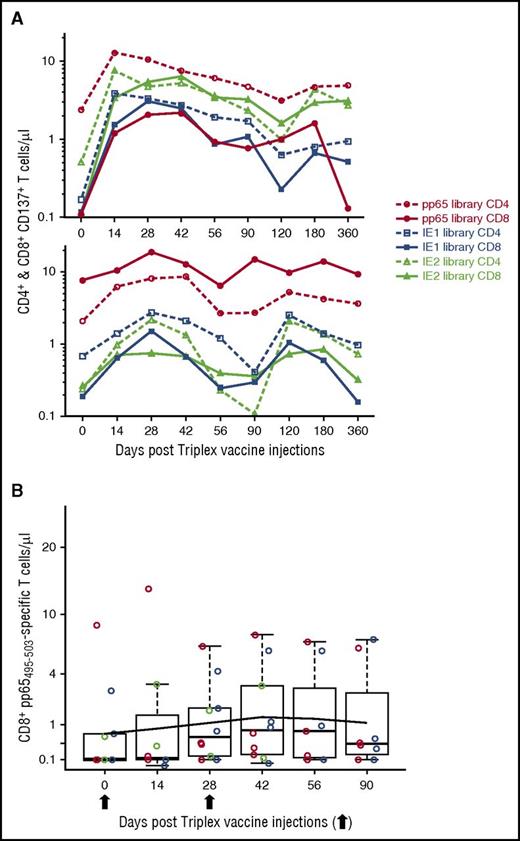
Concentrations of prevaccination CMV-specific CD137+ CD4+ and CD8+ T cells varied widely among participants.15,33 The pp65 library was recognized above the limit of detection (LOD) by 19 (79%) participants, compared with 17 (71%) for the IE1-exon4 library, and 16 (67%) for the IE2-exon5 library.13,15 Baseline CMV-specific T-cell concentrations ranged from 0.1 cells/µL (LOD) to 16.2 cells/µL (for pp65-specific CD137+ CD4+ T cells). Median baseline levels of pp65-, IE1-exon4-, and IE2-exon5-specific CD137+ CD8+ T cells were 0.23, 0.14, and 0.1 cells/µL, respectively. Corresponding median levels for CD137+ CD4+ T cells were 1.3, 0.26, and 0.25 cells/µL.
A broad increase in pp65-, IE1-exon4-, and IE2-exon5-specific CD137+ CD4+ and CD8+ T-cell concentration was noted post-Triplex vaccination. In contrast, a significantly lower number of subjects (N = 3; P < .00001) had no response to any of the 3 CMV antigens expressed by the vaccine (Table 3). Responses to the pp65 portion of the vaccine were recorded in >80% of the participants and were highly significant (P < .00001), whereas those to both IE1 and IE2 were less substantial and infrequent.
Triplex postvaccination responses
| . | pp65 . | IE1-exon4 . | IE2-exon5 . | No response . |
|---|---|---|---|---|
| DL1 (N = 8) | 7 | 0 | 2 | 1 |
| DL2 (N = 8) | 7 | 2 | 1 | 0 |
| DL3 (N = 8) | 6 | 2 | 2 | 2 |
| All (N = 24) | 20 | 4 | 5 | 3 |
| P value | <.00001 | .39 | .59 | .0002 |
| . | pp65 . | IE1-exon4 . | IE2-exon5 . | No response . |
|---|---|---|---|---|
| DL1 (N = 8) | 7 | 0 | 2 | 1 |
| DL2 (N = 8) | 7 | 2 | 1 | 0 |
| DL3 (N = 8) | 6 | 2 | 2 | 2 |
| All (N = 24) | 20 | 4 | 5 | 3 |
| P value | <.00001 | .39 | .59 | .0002 |
Numbers in the first 3 columns represent Triplex responders, defined as subjects who showed a persistently higher than baseline concentration of pp65-, IE1-exon4, or IE2-exon5-specific CD137+ CD8+ or CD4+ T cells through day 360. “No response” column indicates number of subjects who did not respond to any of the 3 CMV proteins expressed by Triplex vaccine. The first 3 rows show data for each of the DL cohorts. The “All” row combines the data for the three DL. In detail, the chance that an individual is counted as a responder under the null-effect hypothesis is 1 in 9. A subject was classified as a responder if either CD8+ or CD4+ measurements were all higher than baseline, yielding a null-hypothesis probability of 0.21 (independent chances being conservative for our purpose). The first 3 P values are the probability of counts as high as or higher than observed, assuming 24 independent subjects with the null probability. The last P value is the analogous probability that as many or fewer subjects failed to respond to any of the 3 libraries, taking the null hypothesis probability (of failing 6 assessments) to be 0.493 for each subject.
Longitudinal analysis of CMV-specific T-cell expansion
The longitudinal analysis was performed by measuring median T-cell concentrations in all subjects and mean concentrations in each DL cohort. In addition, by GEE methods, we used a piecewise linear longitudinal model of fitted means for all participants. Figure 2A shows the concentrations of pp65-specific CD137+ CD8+ (upper panel) and CD137+ CD4+ (lower panel) T cells during the study observation period. The median concentration of pp65-specific CD137+ CD8+ T cells rapidly rose from 0.23 at baseline (day 0) to 3.5 cells/µL at day 42, 2 weeks postvaccination regimen (P = .0003). The piecewise linear model of fitted means estimated a highly significant rise from 1.2 to 3.4 cells/µL in the same time interval (z score [number of standard deviations the observation was above the mean] = 5.4, P < .00001). Concentrations of CD137+ CD4+ T cells were also significantly expanded from a median of 1.3 to a median of 5.2 cells/µL at day 42 (P = .001). Likewise, fitted means rose from 1.9 to 4.3 cells/µL at day 42 (z = 7.6, P < .00001). Expansion of pp65-specific T cells was durable and estimated (by GEE) to remain elevated above prevaccination levels until at least day 360 for pp65-specific CD137+ CD8+ T cells, and at least until day 482 postvaccination for pp65-specific CD137+ CD4+ T cells. Postvaccination increases in pp65-specific T-cell concentrations were substantial and durable in all 3 original CMV-seronegative participants, with 2 subjects being generally above the average for all study participants and the third one with a clear rise, after the second injection (Figure 2A-B; supplemental Figure 1). For all 3 subjects, pp65-specific responses were elevated above baseline at all postvaccination time points (P = .0014 under the null hypothesis of flat response distributions).
pp65-specific T-cell expansion. (A) Postvaccination levels of pp65 CD8 and CD4 T cells. In the box plots, red circles indicate DL1; green circles indicate DL2; blue circles indicate DL3. Boxes cover central 50% of observations, and the central bars show median; whiskers extend to at most 1.5 times box length with more extreme observations shown individually (colored circles). Colored lines represent means for each DL, and black dotted lines show the fitted means (referred as “Model” in the upper right box) on a square-root scale from a piecewise linear GEE model, with a change in slope at day 42 and no DL distinction. Thinner lines indicate the responses profiles for the 3 CMV seronegatives enrolled in the study. UPN 14 (DL2) and 18 (DL3) remained CMV seronegative until day 180, but tested CMV seropositive at day 360. UPN 13 (DL2) received smallpox vaccination. Arrows indicate Triplex vaccine injection. (B) Representative dot plots and gating hierarchy. In the top row, the primary gate set on lymphocytes by use of forward and side scatter is shown. Histogram plots indicate (from left to right) the CD8 (fluorescein isothiocyanate [FITC] conjugated) gating, and the subsequent gating of CD8+ CD137+ (allophycocianin [APC] conjugated) T cells from CMV-seronegative UPN 14, stimulated with the pp65 library on day 180 post-Triplex vaccination (as indicated by the arrow). In the upper right gate of each dot plot, the percentage of CD8+ CD137+ T cells in pp65 stimulated (upper dot plots) and unstimulated (lower dot plots) PBMC samples are shown collected at the post-Triplex vaccination days indicated.
pp65-specific T-cell expansion. (A) Postvaccination levels of pp65 CD8 and CD4 T cells. In the box plots, red circles indicate DL1; green circles indicate DL2; blue circles indicate DL3. Boxes cover central 50% of observations, and the central bars show median; whiskers extend to at most 1.5 times box length with more extreme observations shown individually (colored circles). Colored lines represent means for each DL, and black dotted lines show the fitted means (referred as “Model” in the upper right box) on a square-root scale from a piecewise linear GEE model, with a change in slope at day 42 and no DL distinction. Thinner lines indicate the responses profiles for the 3 CMV seronegatives enrolled in the study. UPN 14 (DL2) and 18 (DL3) remained CMV seronegative until day 180, but tested CMV seropositive at day 360. UPN 13 (DL2) received smallpox vaccination. Arrows indicate Triplex vaccine injection. (B) Representative dot plots and gating hierarchy. In the top row, the primary gate set on lymphocytes by use of forward and side scatter is shown. Histogram plots indicate (from left to right) the CD8 (fluorescein isothiocyanate [FITC] conjugated) gating, and the subsequent gating of CD8+ CD137+ (allophycocianin [APC] conjugated) T cells from CMV-seronegative UPN 14, stimulated with the pp65 library on day 180 post-Triplex vaccination (as indicated by the arrow). In the upper right gate of each dot plot, the percentage of CD8+ CD137+ T cells in pp65 stimulated (upper dot plots) and unstimulated (lower dot plots) PBMC samples are shown collected at the post-Triplex vaccination days indicated.
Alternatively, IE1-exon4- and IE2-exon5-specific T-cell expansions were often noted among participants (Figure 3A), although less consistently and generally of smaller magnitude. Postvaccination median concentrations were not significantly enhanced for IE1-exon4-specific T cells, whereas significant expansions were recorded for IE2-exon5-specific T cells. Specifically, IE2-exon5-specific CD137+ CD8+ T cells had a baseline median of 0.1, which rose to a median of 0.295 cells/µL at day 42 (P = .014). IE2-exon5-specific CD137+ CD4+ T cells’ baseline median rose from 0.245 to 0.750 cells/µL at day 42 (P = .003). Interestingly, there was no significant difference among DLs (P = .69 and P = .13, respectively, for CD137+ CD8+ T cells and CD137+ CD4+ T cells); previous smallpox vaccination (P = .4 and P = .48, respectively), or CMV serostatus (P = .34 and P = .89, respectively) in the responses to all 3 CMV libraries. The lack of a significant difference based on CMV serostatus could be related to the majority of participants being CMV seropositive and relatively insensitive to vaccine amount, albeit most did respond with elevated T-cell levels to at least a single antigen (see Discussion for further comments).
CMV-specific T-cell responses. (A) IE1-exon4- and IE2-exon5-specific T-cell responses. UPN 24 (top plot) and UPN 9 (lower plot) represent longitudinal profiles, showing expansion of the 3 CMV antigens expressed by Triplex. Two injections of Triplex were administered on days 0 and 28. (B) CD8+ pp65495-503-specific T-cell levels. Box plots cover central 50% of observations, and the central bars show median. Whiskers extend to at most 1.5 times box length, and individual observations are shown with colored circles. Red circles indicate subjects from DL1; green indicates subjects from DL2, and blue indicates subjects from DL3. The black line shows the fitted means on a square-root scale from a piecewise linear GEE model, with a change in slope at day 42 and no DL distinction.
CMV-specific T-cell responses. (A) IE1-exon4- and IE2-exon5-specific T-cell responses. UPN 24 (top plot) and UPN 9 (lower plot) represent longitudinal profiles, showing expansion of the 3 CMV antigens expressed by Triplex. Two injections of Triplex were administered on days 0 and 28. (B) CD8+ pp65495-503-specific T-cell levels. Box plots cover central 50% of observations, and the central bars show median. Whiskers extend to at most 1.5 times box length, and individual observations are shown with colored circles. Red circles indicate subjects from DL1; green indicates subjects from DL2, and blue indicates subjects from DL3. The black line shows the fitted means on a square-root scale from a piecewise linear GEE model, with a change in slope at day 42 and no DL distinction.
MHC class I multimer binding assays showed that baseline concentrations of CD8+ T cells specifically binding to the immunodominant pp65495-503 epitope were highly correlated with those of CD137+ CD8+ T cells specific for the whole pp65 peptide library (“r” = 0.9874), in HLA A*0201-positive participants (N = 10; supplemental Table 1, Figure 3B).36,37 Importantly, GEE analysis (Figure 3B) showed a significant (z = 2.5; P = .011) increase in concentration of multimer-binding pp65495-503-specific CD8+ T cells from day 0 to day 42 post-Triplex vaccination, with no significant difference among DL (P = .52) or previous smallpox vaccination (P = .51). These outcomes were remarkably similar to those obtained measuring pp65-specific CD137+ CD8+ T-cell concentrations following Triplex vaccination.37,38 Among the CMV-seronegative subjects, only UPN 18 (supplemental Table 1) was HLA A*0201 positive and was included in the analysis (Figure 3B). On day 28, after 1 Triplex vaccination, we measured 0.8 CD8+ T-cells/µL specific for the pp65495-503 HLA A*0201 epitope; however, at later time points, multimer binding became undetectable.
Functionality and vaccination impact on the T-cell memory compartment
We performed a longitudinal analysis (day 0, 28, 56, 120, 360) of IFN-γ concentrations and memory phenotype on CD8+ and CD4+ T cells stimulated with pp65, IE1-exon4, and IE2-exon5 peptide libraries.39 Triplex vaccinations induced significant expansion of pp65-specific IFN-γ+ CD8+ T cells, indicating that vaccine was able to expand a functional subset of CMV-specific T cells, even in the absence of CMV viremia, which has not been achieved in healthy adults with previous CMV vaccines (Figure 4A upper panel).33,40,41 In particular, median concentration of pp65-specific IFN-γ+ CD8+ T cells was 0.18 at baseline and expanded to 0.44 on day 28 (P = .024), 0.74 on day 56 (P = .003), 0.50 on day 100 (P = .011), and 0.40 cells/µL on day 360 (P = .085). For pp65-specific IFN-γ+ CD4+ T cells, the median concentration rose from 0.18 at baseline to 0.36 cells/µL at day 28, but the rise was not statistically significant (P = .25). Concentrations of IE1-exon4- and IE2-exon5-specific IFN-γ+ T cells did not appreciably expand post-Triplex vaccination.
CMV-specific memory phenotypes and MVA vector-specific responses. (A) Memory phenotype of CMV-specific T cells following Triplex vaccination. The top panel shows representative FACS plots (from UPN 13; gray boxes in the subsequent panels indicate the selected time point) relative to the gating hierarchy used for the memory phenotype analysis of IFN-γ producing T cells. From left to right, lymphocytes gated by use of forward and side scatter; histogram plots indicating the CD3 (peridinin chlorophyll protein Cychrome 5 [PerCP Cy5.5] conjugated) and the CD8 (violet laser excited coumarin dye [V450] conjugated) gating; subsequent gating of CD8+ IFN-γ+ (APC conjugated) T cells unstimulated or stimulated with the pp65 library (as specified on the plot title), and respective percentages shown in the upper right gate of each contour plot. By gating pp65-specific CD8+ IFN-γ+ T cells, 4 subpopulations were identified according to the expression of CD28 and CD45RA (far right dot plot). CD45RA+ CD28+ cells were classified as naive (upper right quadrant); CD45RA− CD28+ cells were classified as central memory (TCM, lower right quadrant), and CD28− cells were classified as effector. Within the effector T-cell group, 2 subpopulations were identified: CD45RA− CD28− (TEM, T effector memory, lower left quadrant) and CD45RA+CD28− (TEMRA, revertant T-effector memory re-expressing CD45RA, upper left quadrant) T cells. The middle panel of line graphs shows the longitudinal profiles of levels of CMV-specific CD8+ and CD4+ T cells producing IFN-γ in CMV-seronegative UPN 13, UPN 14, and UPN 18 following stimulation with the peptide library indicated in the legend. The lower panel of line graphs shows the percentages of the respective memory phenotypes indicated in the legend for CMV-specific CD8+ IFN-γ+ T cells. Memory phenotypes were analyzed when CMV-specific IFN-γ production by T cells in response to a CMV-specific library was ≥0.2%. UPN 13 had been vaccinated against smallpox. Arrows show time of vaccinations (days 0 and 28). The star symbol indicates that UPN 14 and UPN 18 seroconverted and were tested to be CMV seropositive on day 360. (B) T-cell response to the MVA vector. Box plots show levels of vaccinia-specific CD8 T cells on a square-root scale. Colored lines indicate DL and the fitted means are shown as detailed in Figure 2. (C) MVA-specific neutralizing antibodies. The right plot shows MVA specific NT for DL2 and DL3 cohorts, expressed as inhibition dilution (ID50), which is the serum dilution that caused 50% reduction in fluorescence. Box plots cover central 50% of observations, and the central bars show median; whiskers extend to at most 1.5 times box length. All patients are individually shown by a line, as specified in the legend; “pre72” indicates that the subject received smallpox vaccination being born before 1972, during the compulsory smallpox vaccination campaign. The percentage of infection neutralization (ID inhibition dilution) was calculated as follows: (1 − [percentage of fluorescence in Epstein-Barr virus transformed lymphoblastoid cell line cells incubated with serum from vaccinated subject/[percentage of fluorescence in untreated Epstein-Barr virus transformed lymphoblastoid cell line controls]) × 100. Dilutions for each sample ranged from 1:20 to 1:1000. The ID50 is the serum dilution that caused 50% reduction in fluorescence and was calculated by determining the linear slope of the graph plotting ID versus serum dilution by using the next higher and lower ID values that were closest to 50% neutralization.
CMV-specific memory phenotypes and MVA vector-specific responses. (A) Memory phenotype of CMV-specific T cells following Triplex vaccination. The top panel shows representative FACS plots (from UPN 13; gray boxes in the subsequent panels indicate the selected time point) relative to the gating hierarchy used for the memory phenotype analysis of IFN-γ producing T cells. From left to right, lymphocytes gated by use of forward and side scatter; histogram plots indicating the CD3 (peridinin chlorophyll protein Cychrome 5 [PerCP Cy5.5] conjugated) and the CD8 (violet laser excited coumarin dye [V450] conjugated) gating; subsequent gating of CD8+ IFN-γ+ (APC conjugated) T cells unstimulated or stimulated with the pp65 library (as specified on the plot title), and respective percentages shown in the upper right gate of each contour plot. By gating pp65-specific CD8+ IFN-γ+ T cells, 4 subpopulations were identified according to the expression of CD28 and CD45RA (far right dot plot). CD45RA+ CD28+ cells were classified as naive (upper right quadrant); CD45RA− CD28+ cells were classified as central memory (TCM, lower right quadrant), and CD28− cells were classified as effector. Within the effector T-cell group, 2 subpopulations were identified: CD45RA− CD28− (TEM, T effector memory, lower left quadrant) and CD45RA+CD28− (TEMRA, revertant T-effector memory re-expressing CD45RA, upper left quadrant) T cells. The middle panel of line graphs shows the longitudinal profiles of levels of CMV-specific CD8+ and CD4+ T cells producing IFN-γ in CMV-seronegative UPN 13, UPN 14, and UPN 18 following stimulation with the peptide library indicated in the legend. The lower panel of line graphs shows the percentages of the respective memory phenotypes indicated in the legend for CMV-specific CD8+ IFN-γ+ T cells. Memory phenotypes were analyzed when CMV-specific IFN-γ production by T cells in response to a CMV-specific library was ≥0.2%. UPN 13 had been vaccinated against smallpox. Arrows show time of vaccinations (days 0 and 28). The star symbol indicates that UPN 14 and UPN 18 seroconverted and were tested to be CMV seropositive on day 360. (B) T-cell response to the MVA vector. Box plots show levels of vaccinia-specific CD8 T cells on a square-root scale. Colored lines indicate DL and the fitted means are shown as detailed in Figure 2. (C) MVA-specific neutralizing antibodies. The right plot shows MVA specific NT for DL2 and DL3 cohorts, expressed as inhibition dilution (ID50), which is the serum dilution that caused 50% reduction in fluorescence. Box plots cover central 50% of observations, and the central bars show median; whiskers extend to at most 1.5 times box length. All patients are individually shown by a line, as specified in the legend; “pre72” indicates that the subject received smallpox vaccination being born before 1972, during the compulsory smallpox vaccination campaign. The percentage of infection neutralization (ID inhibition dilution) was calculated as follows: (1 − [percentage of fluorescence in Epstein-Barr virus transformed lymphoblastoid cell line cells incubated with serum from vaccinated subject/[percentage of fluorescence in untreated Epstein-Barr virus transformed lymphoblastoid cell line controls]) × 100. Dilutions for each sample ranged from 1:20 to 1:1000. The ID50 is the serum dilution that caused 50% reduction in fluorescence and was calculated by determining the linear slope of the graph plotting ID versus serum dilution by using the next higher and lower ID values that were closest to 50% neutralization.
It has been shown that pp65-, IE1-, and IE2-specific T cells have a predominantly differentiated memory phenotype that is characterized by loss of membrane expression of the costimulatory molecule CD28 and re-expression of the RA isoform of CD45 (CD28− CD45RA+).42-44 Percentages of CD28− CD45RA+ (effector memory “revertant” T cells, TEMRA) along with CD28+ CD45RA+ (naive T cells), CD28+ CD45RA− (central memory T cells, TCM), and CD28− CD45RA− (effector memory T cells, TEM) widely varied among participants before and after Triplex vaccination, although predominance of the TEMRA was constantly detected.42-44 TEMRA accounted for 45% of pp65 IFN-γ+ CD8+ T-cell phenotypes, compared with 23% for TCM, 19% for TEM, and 14% for naive T cells. We found marked postvaccination TEMRA increase in the CD8+ IFN-γ+ pp65-specific T-cell compartment of CMV-seronegative subjects (Figure 4A). Interestingly, for UPN 14 and UPN 18, who seroconverted and were tested CMV-seropositive at study end (day 360), a noticeable increase in IFN-γ+ pp65-specific CD8+ TEMRA and a steep surge of IFN-γ+ IE1- and IE2-specific CD8+ TEMRA cells were detected at the presumed time of seroconversion (Figure 4A).
Cellular and humoral response to the MVA vaccine vector
Triplex induced significant vaccinia-specific T-cell increases by day 42 (GEE z score 3.5; P = .0005), with an estimated decline to prevaccination levels at day 274 (Figure 4B). In further analogy with the CMV-specific T-cell response, we did not find a Triplex DL response effect in the study population or a response difference in subjects who previously received smallpox vaccination. This observation suggests repeated administration of the vaccine might be feasible without overwhelming antivector immunity interfering with vaccine function.
We measured levels of MVA-specific neutralizing antibodies in sera of both DL2 and DL3 cohorts (Figure 4C, supplemental Figure 2). Two of 4 subjects who were born before 1972 (vaccinated with live vaccinia virus) had detectable neutralizing titers (NT) pre-Triplex vaccination (Figure 4C, supplemental Figure 2). In 1 participant (UPN 17, DL3), a marked anamnestic response occurred. Interestingly, UPN 17 had also potent CMV-specific T-cell expansions (supplemental Figure 2A) and is also the only subject who had self-resolved grade 3 erythema (Table 1). All participants developed MVA-specific NT by day 42 (P = .014). In the DL3 cohort, there was a significant increase in MVA-specific NT by day 14 (P = .022), although surprisingly by day 42, DL2 and DL3 MVA NT no longer differed (P = .46).
Mobilization of Epstein-Barr virus (EBV)–specific T-cell responses
We investigated whether the CMV-pp65-specific postvaccination T-cell expansion could be attributed to a nonspecific inflammatory response. IFN-γ production, as a result of stimulation by peptide libraries from immunodominant EBV T-cell target antigens, was tested in 6 subjects (2 for each Triplex DL).45,46 By using intracellular cytokine staining and Enzyme-linked ImmunoSpot, we did not find increases in IFN-γ at the day 56 time point compared with prevaccination for both Epstein-Barr nuclear antigen 1 and latent membrane protein 2 peptide libraries after 2 Triplex injections (day 28 and day 56; supplemental Figure 3). Although limited to a subgroup of study participants and to a single herpesvirus of which the majority of adults are infected,47 these data indicate that Triplex vaccination did not induce an inflammatory response resulting in the activation of memory T cells from the related herpesvirus EBV.48
Discussion
This study reports the first clinical evaluation of Triplex, a recombinant (r) MVA expressing CMV antigens that are associated with protective immunity. The phase 1 clinical trial data indicate that the vaccine is well tolerated in healthy adults. The major finding of this study, which has broad clinical implications, is that Triplex is highly immunogenic, and expansion of durable CMV-specific T cells was observed in both CMV-seropositive and -seronegative participants, and in those subjects who previously received smallpox vaccination.48 These properties make the vaccine a superior candidate for further evaluation in many patient populations regardless of CMV serostatus and prior exposure to poxviruses.
The excellent tolerability profile of healthy adults to the vaccine (Table 1) is in agreement with previous phase 1 studies of other MVA candidate vaccines, which were well tolerated in immunosuppressed and nonimmunosuppressed patient populations.19,26-29 MVA is often administered intradermally, although we chose the IM route for Triplex, because this route is favored in worldwide immunization programs due to the reduced injection site pain, adverse reactions, and technical ease.49 Furthermore, studies have shown that both intradermal and IM routes are comparable in inducing cellular immunity.49 The absence of any cardiac AE in any participants who received Triplex vaccination confirms previous studies, indicating that MVA has no adverse impact on the cardiovascular system, both in healthy adults and in HCT recipients.28,29,50 It has been shown that MVA viral DNA rapidly decays, does not cause organ toxicity, and does not integrate to the host genome.31,32 Our study confirms previous reports: MVA viral DNA was undetectable for all vaccinees, except for minimal residual levels detected in 2 participants from the DL3 cohort. Heterologous immunity with recruitment of T cells of promiscuous specificities to inflammatory sites has been documented for vaccinia virus and other pathogens.48 Although limited in scope, our results suggest that Triplex vaccination did not cause the expansion of T cells recognizing EBV antigens, a related herpes virus to CMV. Minimal increase in the production of the proinflammatory cytokine IFN-γ was observed for EBV-specific T cells, following the Triplex vaccination regimen. These data are in agreement with studies indicating the limited inflammatory response induced by MVA vaccination.17-21
CMV viremia protection is dependent upon the reconstitution of CMV-specific T-cell subsets in HCT recipients4-8 ; consequently, Triplex immunogenicity evaluation focused on magnitude and quality of CMV-specific CD4 and CD8 T-cell expansion. We chose the CD137 assay as a reliable method to estimate magnitude and duration of the CMV-specific T-cell expansion after Triplex vaccination. Immune-monitoring of ex vivo T-cell responses has been successfully performed with this surrogate marker of T-cell activation.33,42,43 Although undetectable on unstimulated T cells, the CD137 marker becomes uniformly upregulated 24 hours after stimulation on virtually all responding T cells, regardless of differentiation stage or profile of cytokine secretion,51 thus providing a comprehensive tool for the immunological assessment of Triplex vaccine. Once we established that the vaccine induced durable expansion of CMV specific T cells, we next assessed Triplex impact on functionality, by using intracellular staining for IFN-γ,52 and surface markers associated with memory phenotype. Although the large majority of CMV-specific T cells produce IFN-γ, and CMV-specific memory phenotype assessments have been typically performed on IFN-γ producing T cells,35,36,44 a proportion of CMV-specific T cells do not produce this cytokine. It has been previously established that the production of this cytokine may differ based on the CMV antigen and T-cell subset.15,36 The functional signature of CMV-specific T cells that protect against CMV reactivation in the allo-HCT setting is incompletely understood, although studies suggest that polyfunctional CD8+ T cells may be more effective in controlling CMV viremia.6,10 Thus, future studies in the HCT setting should include the evaluation of a larger panel of cytokines and multiple functions to comprehensively characterize Triplex immunogenicity.
T-cell immune responses induced by Triplex at all DL reached unprecedented breadth and magnitude, not observed with prior CMV vaccine candidates (Table 3; Figure 2).33,41,44 A canarypox vector expressing CMV pp65 (ALVAC-pp65 vaccine) induced T-cell responses only in CMV-seronegative subjects.41 Responses in CMV-seropositive healthy adults were either low or detectable only after in vitro stimulation for both CMVPepVax peptide vaccine and the TransVax (Astellas Pharma Inc, Tokyo, Japan) CMV DNA vaccine.33,40 In a CMV-seropositive healthy adult, a subunit CMV vaccine likely targets the TCM compartment, which is under homeostatic control and therefore difficult to alter.53 Nevertheless, by using Triplex vaccine, it was possible to achieve a long-lasting significant boosting of pp65-specific T-cell natural immunity. Recombinant viral vector vaccines generally provide superior immunogenicity and enhanced durability, compared with peptide and DNA-based vaccines, which often require adjuvant and are labile.54,55 In contrast to ALVAC, early and late transcription are unimpaired in MVA, which results in a longer duration of antigen production in rMVA-infected cells, leading to enhanced immunogenicity of rMVA-based vaccines.56 In addition, levels of pp65495–503–specific CD8+ T cells significantly increased postvaccination in HLA A*0201 participants, with a kinetic pattern similar (Figures 2A and 3B) to the CD137+ CD8+ T-cell–specific expansion for the whole pp65 peptide library. These data suggest that Triplex vaccination does not alter the T-cell native host recognition patterns of immunodominant CMV antigens measured in CMV-seropositive individuals.15,33,57 The promising results in CMV-seropositive healthy subjects are of relevance given the intended use of this vaccine in CMV-seropositive HCT recipients, at highest risk for CMV reactivation, which is a cause of increased transplant-related mortality.2,3 The CMV seropositivity rate in the last 5 years for COH HCT patients, who mainly reside in LAC, has been 85% (including adults over 21 years of age), thus making our study population representative of the national HCT population.
Donor-derived virus-specific T cells (VST) have shown promise in treating refractory CMV and other viral infections in solid organ transplant and HCT recipients, and when a donor is unavailable, third party, partially matched allogeneic T cells, have also shown efficacy.58,59 Some investigators have reported that VST lines cannot be obtained without the donor’s previous virus exposure,60 whereas more recently it has been demonstrated the feasibility of deriving CMV-specific T cells from seronegative patients for T-cell therapy.61 Naive T cells primed to recognize CMV showed efficacy, although they were restricted to atypical epitopes and did not recognize the immunodominant pp65495-503 epitope, in contrast to most HLA A*0201 CMV-seropositive donors.61 Data from our trial indicated that pp65495-503–specific CD8 T cells were detected in UPN 18, the only CMV seronegative that was typed as HLA A*0201, 28 days after the first Triplex injection. Further studies in CMV-seronegative subjects would be required to address whether atypical CMV-pp65 epitope recognition is generated in CMV-naive subjects vaccinated with Triplex. Based on the durable primary immune response elicited in CMV-seronegative participants (Figure 2A) post-vaccination, Triplex vaccination of CMV-seronegative donors could provide an alternative strategy to rapidly generate CMV-specific VST for adoptive T-cell infusions.62 This significant property also has value in many clinical contexts, including in solid organ transplant, when it is desirable to induce primary CMV immunity in patients who are naive to the virus and at enhanced risk for CMV uncontrolled viremia and late CMV disease.63
Although ex vivo IE1 exon4 and IE2 exon5 CD137+ T-cell–specific expansions were detected in some participants (Figure 3A), there was no increase in IFN-γ+ production, and the number of responders to IE1-exon4 and IE2-exon5 was much lower than to pp65 (Table 3). The reduced response may be due to the nonviremic status of the healthy population. The IE1 and IE2 proteins are among the first to be expressed during CMV infection and reactivation. These proteins are indispensable for viral replication and may therefore be particularly valuable targets for a vaccine-based strategy against CMV viremia.15,64,65 However, a significant expansion of functional IE1- and IE2-specific T-cell subsets may not be consistently reached by Triplex vaccination when CMV viremia is absent, as in the case of healthy subjects. In addition, far fewer healthy subjects have an ongoing CMV-specific T-cell response that includes recognition of the IE1 and IE2 antigens compared with pp65.15
Strong predominance of long-lived and functional TEMRA cells has been reported during primary CMV infection, because these cells are critical for viremia control.35,36,44,66 It is interesting to note that the profile of CMV-seronegative participant UPN 14, who tested CMV-seropositive at day 360, showed that levels of IFN-γ+ TEMRA cells (Figure 4A) increased postvaccination, reaching an apex when the individual seroconverted. Our limited set of data suggests that Triplex vaccination may simulate a CMV primary infection with the induction of functional long-lasting CMV-specific T cells associated with effective control of CMV viremia.
Investigations of the durability of immunity following smallpox vaccination showed that vaccinia-specific antibodies may persist up to 75 years postvaccination, whereas T-cell responses have a half-life of 8 to 15 years.67 Data from the current trial showed similar trends. Participants who received previous smallpox vaccination (N = 6) had minimal prevaccination vaccinia-specific T cells; in contrast, 2 of 4 tested participants born before 1972 had appreciable titers of vaccinia-specific neutralization antibodies. Although significant increases of both humoral and cellular vaccinia-specific T-cell responses were elicited by Triplex vaccination (Figure 4B-C), they did not prevent robust expansions of CMV-specific T-cell responses (Table 3, Figure 2). Importantly, there was no effect of previous smallpox vaccination on Triplex driven expansion of CMV-specific T cells. Our data do not support the contention that prior antivaccinia immunity renders rMVA vector vaccines ineffective.48
The favorable safety and immunogenicity outcomes of this study in healthy adults paved the way for an ongoing phase 2 randomized, blinded, and placebo-controlled multicenter trial (NCT02506933; www.clinicaltrials.gov) to evaluate protective function of Triplex vaccine, in CMV-positive patients undergoing HCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The administrative assistance of Peter Kwon, Supriya Bautista, and Aline Matsuo are gratefully acknowledged. The authors would like to thank the COH employees who volunteered for the study and the safety monitoring board members. The authors wish to acknowledge a gift of purified rabbit anti-vaccinia sera that was manufactured by Quality Biological Inc (Gaithersburg, MD) under contract HHSN272201100023C issued by the Vaccine Research Program, Division of AIDS, National Institute of Allergy and Infectious Diseases.
This study was supported by grants from the National Institutes of Health, National Cancer Institute R01-CA77544 (D.J.D.) and P30-CA033572 to the COH Comprehensive Cancer Center.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: D.J.D., J.L., J.A.Z., and C.L.R. developed the study design; J.A.Z., R.N., I.A., S.D., J.D., and M.C. contributed to the clinical implementation of the study, supervision, scheduling of the study participants and vaccine administration; J.L. designed and did the final statistical analysis and verified its accuracy; Q.Z., J.M., T.I.K., W.T., F.W., F.C., N.H. and C.L.R. performed the laboratory experiments; C.L.R. did the immunological analyses; C.L.R., D.J.D., and J.L. wrote the manuscript. All authors critically revised, reviewed, and approved the final version of this study.
Conflict–of-interest disclosure: J.A.Z. received royalty funding from Astellas Pharma/Vical Inc. D.J.D. is chair of the Scientific Advisory Board and receives personal service fees from Helocyte Inc. No funds from a commercial entity were used to support any aspect of this study. All other authors declare no competing financial interests.
Correspondence: Don J. Diamond, Department of Experimental Therapeutics, Beckman Research Institute of City of Hope, 1500 E. Duarte Rd, Fox South, Duarte, CA 91010; e-mail: ddiamond@coh.org.
References
Author notes
C.L.R. and J.L. contributed equally to this study.

![Figure 2. pp65-specific T-cell expansion. (A) Postvaccination levels of pp65 CD8 and CD4 T cells. In the box plots, red circles indicate DL1; green circles indicate DL2; blue circles indicate DL3. Boxes cover central 50% of observations, and the central bars show median; whiskers extend to at most 1.5 times box length with more extreme observations shown individually (colored circles). Colored lines represent means for each DL, and black dotted lines show the fitted means (referred as “Model” in the upper right box) on a square-root scale from a piecewise linear GEE model, with a change in slope at day 42 and no DL distinction. Thinner lines indicate the responses profiles for the 3 CMV seronegatives enrolled in the study. UPN 14 (DL2) and 18 (DL3) remained CMV seronegative until day 180, but tested CMV seropositive at day 360. UPN 13 (DL2) received smallpox vaccination. Arrows indicate Triplex vaccine injection. (B) Representative dot plots and gating hierarchy. In the top row, the primary gate set on lymphocytes by use of forward and side scatter is shown. Histogram plots indicate (from left to right) the CD8 (fluorescein isothiocyanate [FITC] conjugated) gating, and the subsequent gating of CD8+ CD137+ (allophycocianin [APC] conjugated) T cells from CMV-seronegative UPN 14, stimulated with the pp65 library on day 180 post-Triplex vaccination (as indicated by the arrow). In the upper right gate of each dot plot, the percentage of CD8+ CD137+ T cells in pp65 stimulated (upper dot plots) and unstimulated (lower dot plots) PBMC samples are shown collected at the post-Triplex vaccination days indicated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/1/10.1182_blood-2016-07-729756/4/m_blood729756f2.jpeg?Expires=1767707593&Signature=2phM2na6Nzq85hQE0pPrY5UiUDYfbuVRB5pDsg45ZgXD~aFDBvNf4iUk8hapVmhnxV5S~YUr5W~YtKFALFXjPJETY73uwKsQ-nD53NEKptMdpt1TpNDsW82iEipm~xts8mG7IatzfigOXqxMdrFaJBQmjaSwG1j7DthyqEOZqRVcSi9xliAVVinx6kUB9fBp1MEJRCu4O9VlknrJwKhZO98J3acgbMA5zNOotw2ifztVfhMN4DGpvcQGBQ6TCYrN~mLnQQpGDWV-9DT~iiFBbcOiAq7IKNmJEOnaml60~nCM5PLRE0x~VRHbQnT2JNyngdE963bRMdXQmW0mSRLKNw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. CMV-specific memory phenotypes and MVA vector-specific responses. (A) Memory phenotype of CMV-specific T cells following Triplex vaccination. The top panel shows representative FACS plots (from UPN 13; gray boxes in the subsequent panels indicate the selected time point) relative to the gating hierarchy used for the memory phenotype analysis of IFN-γ producing T cells. From left to right, lymphocytes gated by use of forward and side scatter; histogram plots indicating the CD3 (peridinin chlorophyll protein Cychrome 5 [PerCP Cy5.5] conjugated) and the CD8 (violet laser excited coumarin dye [V450] conjugated) gating; subsequent gating of CD8+ IFN-γ+ (APC conjugated) T cells unstimulated or stimulated with the pp65 library (as specified on the plot title), and respective percentages shown in the upper right gate of each contour plot. By gating pp65-specific CD8+ IFN-γ+ T cells, 4 subpopulations were identified according to the expression of CD28 and CD45RA (far right dot plot). CD45RA+ CD28+ cells were classified as naive (upper right quadrant); CD45RA− CD28+ cells were classified as central memory (TCM, lower right quadrant), and CD28− cells were classified as effector. Within the effector T-cell group, 2 subpopulations were identified: CD45RA− CD28− (TEM, T effector memory, lower left quadrant) and CD45RA+CD28− (TEMRA, revertant T-effector memory re-expressing CD45RA, upper left quadrant) T cells. The middle panel of line graphs shows the longitudinal profiles of levels of CMV-specific CD8+ and CD4+ T cells producing IFN-γ in CMV-seronegative UPN 13, UPN 14, and UPN 18 following stimulation with the peptide library indicated in the legend. The lower panel of line graphs shows the percentages of the respective memory phenotypes indicated in the legend for CMV-specific CD8+ IFN-γ+ T cells. Memory phenotypes were analyzed when CMV-specific IFN-γ production by T cells in response to a CMV-specific library was ≥0.2%. UPN 13 had been vaccinated against smallpox. Arrows show time of vaccinations (days 0 and 28). The star symbol indicates that UPN 14 and UPN 18 seroconverted and were tested to be CMV seropositive on day 360. (B) T-cell response to the MVA vector. Box plots show levels of vaccinia-specific CD8 T cells on a square-root scale. Colored lines indicate DL and the fitted means are shown as detailed in Figure 2. (C) MVA-specific neutralizing antibodies. The right plot shows MVA specific NT for DL2 and DL3 cohorts, expressed as inhibition dilution (ID50), which is the serum dilution that caused 50% reduction in fluorescence. Box plots cover central 50% of observations, and the central bars show median; whiskers extend to at most 1.5 times box length. All patients are individually shown by a line, as specified in the legend; “pre72” indicates that the subject received smallpox vaccination being born before 1972, during the compulsory smallpox vaccination campaign. The percentage of infection neutralization (ID inhibition dilution) was calculated as follows: (1 − [percentage of fluorescence in Epstein-Barr virus transformed lymphoblastoid cell line cells incubated with serum from vaccinated subject/[percentage of fluorescence in untreated Epstein-Barr virus transformed lymphoblastoid cell line controls]) × 100. Dilutions for each sample ranged from 1:20 to 1:1000. The ID50 is the serum dilution that caused 50% reduction in fluorescence and was calculated by determining the linear slope of the graph plotting ID versus serum dilution by using the next higher and lower ID values that were closest to 50% neutralization.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/1/10.1182_blood-2016-07-729756/4/m_blood729756f4.jpeg?Expires=1767707593&Signature=GVFBRSJjAmuMVtKs2PH1bJrG6q0r2NJIuXXEccQ85~KbetX7NMiU~9kHk2oEpvIgZPDo0IAzkTjbq9i1MJioexm~sFoRfnChOUATn4D5qGm23IN-toSgUc0kYcZ~f3cjOt-zCDGmLYCoMCjPnQMkwg6195S82~b4y064XywgmsvvvcO-wLd8BR73vJ4S~lFj0z4BcJWmlR-biHUGc~NdTVYaS00-fgO4Cu5qmZfW7xtVDO2KTMYtKgTj~q9IozPVfpfjmQk51BQkV63UWpmnycLUzwtGVWpSu9v3za~7s2FsjWrFPV9KFipRgdyDm8VxlC2hQHJhwQXi~0LlRevemA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)