Key Points
The endogenous inhibitors of APC also inhibit other coagulation proteases rendering them unacceptable for treatment of hemophilia.
Rationally designed APC-specific serpins rescue thrombin generation in vitro and restore hemostasis in hemophilia mouse models.
Abstract
Hemophilia is a bleeding disorder caused by deficiency in factors VIII or IX, the two components of the intrinsic Xase complex. Treatment with replacement factor can lead to the development of inhibitory antibodies, requiring the use of bypassing agents such as factor VIIa and factor concentrates. An alternative approach to bypass the Xase complex is to inhibit endogenous anticoagulant activities. Activated protein C (APC) breaks down the complex that produces thrombin by proteolytically inactivating factor Va. Defects in this mechanism (eg, factor V Leiden) are associated with thrombosis but result in less severe bleeding when co-inherited with hemophilia. Selective inhibition of APC might therefore be effective for the treatment of hemophilia. The endogenous inhibitors of APC are members of the serpin family: protein C inhibitor (PCI) and α1-antitrypsin (α1AT); however, both exhibit poor reactivity and selectivity for APC. We mutated residues in and around the scissile P1-P1′ bond in PCI and α1AT, resulting in serpins with the desired specificity profile. The lead candidate was shown to promote thrombin generation in vitro and to restore fibrin and platelet deposition in an intravital laser injury model in hemophilia B mice. The power of targeting APC was further demonstrated by the complete normalization of bleeding after a severe tail clip injury in these mice. These results demonstrate that the protein C anticoagulant system can be successfully targeted by engineered serpins and that administration of such agents is effective at restoring hemostasis in vivo.
Introduction
Hemostasis is a crucial part of the physiological response to tissue damage. When blood components come into contact with extravascular cells and proteins, platelets accumulate and the coagulation cascade is initiated.1 The sequential activation of zymogens to active serine proteases culminates in the formation of the effector serine protease thrombin.2,3 Thrombin activates platelets and cleaves fibrinogen to fibrin, the two major components of a stable blood clot.4,5 Thrombin also cleaves and activates the critical factors VIII (fVIII) and fV, thereby allowing the formation of the highly efficient intrinsic Xase (fVIIIa-fIXa) and prothrombinase (fVa-fXa) complexes, resulting in the burst of thrombin formation necessary to establish and maintain the integrity of the hemostatic clot (supplemental Figure 1, available on the Blood Web site). In addition to these procoagulant activities, thrombin plays a crucial role in downregulating its own formation by activation of the protein C anticoagulation pathway.6 When bound to its cofactor thrombomodulin (TM), thrombin efficiently cleaves protein C to activated protein C (APC), a powerful anticoagulant that proteolytically inactivates fVIIIa and fVa, thereby shutting down the intrinsic Xase and prothrombinase complexes (supplemental Figure 1).
Hemophilia A and hemophilia B are X-linked genetic disorders with rates of 1:5000 and 1:20 000 live male births, respectively.7 The bleeding associated with these disorders is the result of a defect or deficiency in fVIII (hemophilia A) or fIX (hemophilia B), the two components of the intrinsic Xase complex. The mainstream treatment of hemophilia consists of replacing the affected factor on demand when bleeds occur or by using prophylaxis.8-13 Prophylactic treatment is not completely effective and reduces only the frequency of bleeds. Neither treatment regimen prevents hemophilic arthropathy (extravasation of blood into the joints), a major cause of morbidity associated with hemophilia.14 In addition, because the replacement factor is effectively a foreign protein, treatment is often associated with formation of inhibitory antibodies,15,16 which necessitates using a different class of therapeutics termed “bypassing agents.”17 Bypassing agents increase thrombin generation through mechanisms independent of the intrinsic Xase complex, the most commonly used of which are fVIIa (NovoSeven), prothrombin concentrates, and FEIBA. However, these agents suffer from short half-lives and result in variable responses in patients.18-21 They are also less effective than replacement therapy before inhibitor formation and are not commonly used prophylactically.22
Currently all approved bypassing agents improve thrombin generation by bolstering the levels of coagulation factors. An alternative approach is to reduce the efficiency of natural anticoagulant mechanisms (eg, using small interfering RNA to knock down antithrombin levels or an antibody to inhibit tissue-factor pathway inhibitor).23,24 The protein C system is particularly attractive because partial APC resistance reduces the frequency and severity of bleeding in hemophiliacs, with the common fV Leiden variant providing an early proof-of-concept in humans.25-27 The mode of action of an APC inhibitor is to prolong the life-span of the prothrombinase complex, thereby directly increasing thrombin generation at the site of tissue damage (supplemental Figure 1).
The endogenous inhibitors of APC are members of the serpin family.28 Serpins use a well-characterized mechanism of protease inhibition, in which the protease recognizes a stretch of the exposed reactive center loop (RCL) as a substrate (supplemental Figure 2) and is then trapped in a covalent complex after a large conformational change.29 Several serpins are able to inhibit APC, including α1-antitrypsin (α1AT),30 plasminogen activator inhibitor 1,31 and protein C inhibitor (PCI).32 PCI is thought to be the main physiological inhibitor of APC; however, its rate-constant for inhibition of APC is only ∼500 M–1s−1, and it is also capable of inhibiting procoagulant proteases such as thrombin, fXa, and fVIIa.32,33 The other circulating serpins are also inefficient APC inhibitors that lack specificity. It is therefore unlikely that the administration of an endogenous inhibitor of APC would have the desired hemostatic effect. Herein we describe the engineering and characterization of a serpin specific for APC and show that it is effective in increasing thrombin generation in vitro and normalizing hemostasis in hemophilia B mice.
Methods
Protein expression and purification
Human PCI with its N-terminal 21 residues truncated (Δ21, first residue is Ala22) was expressed from Escherichia coli and purified as described34 with some modifications. Full-length human α1AT with M358R and C232S mutations was expressed from a pET SUMO protein expression system (Life Technologies) construct in Rosetta2(DE3)pLysS cells. Protein was purified from clarified cell lysate by using a combination of HiTrap high performance immobilized metal ion affinity chromatography and Q Sepharose columns (GE Healthcare). The SUMO tag was removed and the α1AT was repurified. The human TM extracellular domain, containing a TEV protease cleavage site between EGF domains 3 and 4 was expressed in HEK-EBNA cells. All mutations described were introduced by site-directed mutagenesis using the QuickChange mutagenesis method and verified by sequencing at Source BioScience (Cambridge, United Kingdom). Detailed descriptions of methods for protein production are provided in the supplemental Data.
Determination of inhibition constants
Second-order rate constants of protease inhibition were measured by a discontinuous method under pseudo first-order conditions, using at least a fivefold molar excess of serpin over protease. All reagents were diluted into assay buffer (20 mM tris[hydroxymethyl]aminomethane [Tris; pH 7.4], 100 mM NaCl, 0.2% bovine serum albumin, 0.1% PEG8,000). For reactions with APC and fXa, 2.5 mM CaCl2 was included in the buffer. Reactions were incubated at room temperature and were stopped for each time point by the addition of the chromogenic substrate appropriate for the protease used (S2238 for thrombin, S2366 for APC and fXIa, and S2222 for fXa). The slope of the linear part of A405 (absorbance at 405 nm) over time gave the residual protease activity at each time point. The apparent (observed) first-order rate constant kobs was calculated from the slope of a plot of the natural log of residual protease activity over time. kobs was measured for at least 5 different serpin concentrations and plotted against serpin concentration. The slope of this linear plot gave the second-order rate constant k2. For each determination, the standard error of the slope is given.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis of complex formation
Serpins and human plasma-derived proteases were diluted into 20 mM Tris [pH 7.4] and 100 mM NaCl before the experiment. For reactions with APC and fXa, 2.5 mM CaCl2 was included in the buffer. Serpin and protease were incubated at equimolar concentrations (1 μM final), and samples were removed at the indicated time points. Sodium dodecyl sulfate (SDS) sample buffer was added with (reducing) or without (nonreducing) 50 mM dithiothreitol, and samples were place in a boiling water bath to stop the reaction. Samples were then run on SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and reaction products were visualized by Coomassie staining.
Prothrombin time and activated partial thromboplastin time
Fresh frozen human plasma from 3 different donors was mixed to generate pooled plasma. For the prothrombin time (PT) assays, plasma was diluted twofold in 20 mM Tris [pH 7.4], 100 mM NaCl, and 2.5 mM CaCl2. Then, 50 μL of diluted plasma was mixed with 50 μL of serpin and incubated for 60 seconds, and 100 μL PT reagent (TriniClot PT Excel) was added to initiate the clotting reaction. For activated partial thromboplastin time (aPTT) assays, 50 μL plasma was mixed with 50 μL serpin diluted in 20 mM Tris [pH 7.4] and 150 mM NaCl, 100 μL aPTT reagent (Triniclot automated aPTT reagent) was added and incubated for 5 minutes, and 100 μL 25 mM CaCl2 was added to start the clotting reaction. Time to clot formation for both assays was measured on a Stago STart coagulation analyzer. All reactions were carried out at 37°C and used a final serpin concentration of 5 μM.
Thrombin generation assays
Normal human plasma was purchased from George King Biomedical. To begin, 40 μL plasma was mixed with 10 μL Technothrombin TGA reagent B (tissue-factor low/phospholipids mix, TechnoClone) and 5 μL protein sample diluted in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [pH 7.4], 150 mM NaCl, and 0.1% bovine serum albumin. To initiate the reaction, 50 μL of 1 mM Z-Gly-Gly-Arg-AMC (Bachem) in 15 mM CaCl2 was added. Fluorescence was read by using 360 nm excitation and 460 nm emission wavelengths. Fluorescence units were converted to thrombin concentration by using a thrombin generation assay (TGA) calibration kit (TechnoClone). Data were analyzed by using the Technothrombin TGA evaluation spreadsheet (TechnoClone). All reactions were performed at 37°C.
Tail clip assays
Tail clip assays were performed essentially as described.35 Briefly, male wild-type (wt) or hemophilia B mice (BALB/c) (8 to 16 weeks of age) were anesthetized by using 2% isoflurane at 2 mL/min and weighed. Mice were injected through the tail vein with 200 μL phosphate-buffered saline (PBS) or α1AT variant diluted into PBS. Five minutes after injection, the tail was cut at 3 mm diameter and placed into a tube filled with prewarmed saline solution (37°C); blood was collected for 10 minutes. Collection tubes were centrifuged, and the saline solution was suctioned off. Lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 1 mM EDTA) was added, and red cells were allowed to lyse for 10 minutes at room temperature. Samples were centrifuged, and absorbance of the supernatant at 575 nm was determined to measure hemoglobin content. A standard curve using known volumes of blood was constructed to allow calculation of total blood loss volume.
Intravital microscopy
The real-time in vivo imaging experiments were performed essentially as described.35 Briefly, male hemophilia B mice ∼12 weeks of age were anesthetized, and the cremaster muscle was exposed and stretched across an intravital microscopy tray. Mice were infused with 10 μg rat anti-CD41 Alexa 555–labeled antibody and 10 μg anti-fibrin Alexa 647–labeled antibody in a total volume of 100 μL. This was followed by infusion of 100 μL PBS or 100 μL α1AT variant diluted in PBS. Laser injury was induced in cremaster arterioles by using a pulse-nitrogen dye laser through the microscope objective. Both bright-field and fluorescence images were collected for 3 to 4 minutes. Data were analyzed by using SlideBook 5 software (Intelligent Imaging Innovations).
Results
Engineering specificity
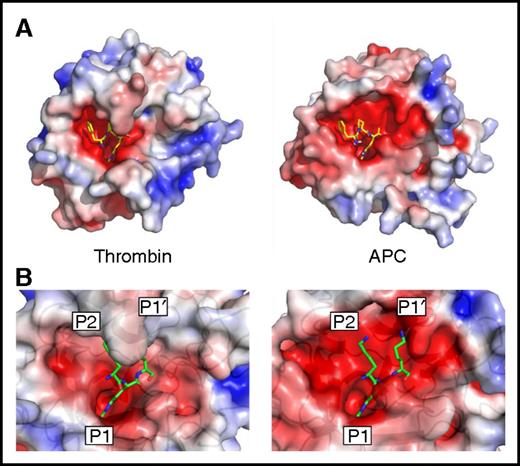
The main determinant of serpin specificity is the RCL sequence in and around the scissile bond. In the standard substrate nomenclature, the scissile bond is denoted P1-P1′, with N-terminal residues numbered P2, P3, and so on and C-terminal residues numbered P2′, P3′, and so on.36 All clotting proteases, including APC, favor an arginine in the P1 position because of Asp189 (chymotrypsin template numbering) at the base of the S1 pocket. We examined the structures of APC and thrombin bound to the same covalent inhibitor (d-Phe-Pro-Arg-chloromethylketone [PPACK]) to see if differences in shape and properties in other parts of the substrate binding site might be exploited to confer specificity for APC (Figure 1A). PPACK binds in an identical fashion in the 2 structures, with the P1 Arg deep in the S1 pocket, the P2 Pro in the S2 pocket, and the d-Phe in the aryl binding site (S4 pocket). However, PPACK is buried in the active site cleft of thrombin, whereas the active site of APC is wide open, especially on either side of the P1 residue (the S2 and S1′ sites). This is primarily the result of the difference in size of the 60-loop in APC and thrombin; APC has a single insertion relative to chymotrypsin and thrombin has a 9-residue insert. The 60-loop in thrombin has been well characterized and acts as a hydrophobic cap that restricts the S2 and S1′ pockets, favoring small hydrophobic amino acids like Gly and Pro at S2 and Ser in S1′.37 In addition, the S1′ site is also more acidic in APC than in thrombin because of the side chain of Asp60a in the position of Lys60h in thrombin. We previously reported a high-resolution crystal structure of the PCI-thrombin complex (3B9F) that revealed the details of the RCL interaction with the active site of thrombin.34 Superposition of APC on thrombin in this structure shows that the features observed with PPACK are largely preserved, namely that APC is permissive of large side chains at the S2 and S1′ sites and would interact favorably with basic residues (Figure 1B). Therefore, we hypothesized that bulky residues at the P2 and P1′ positions would be disfavored by thrombin and that basic residues at these positions might additionally contribute to conferring specificity for APC. This is further supported by comparison of the natural substrates of thrombin and APC (supplemental Table 1).
Active site properties of thrombin and APC. (A) Electrostatic surface representations of human thrombin (1PPB42 ) and human APC (from 1AUT44 ), with the small molecule inhibitor PPACK bound into the active site cleft (sticks). (B) Zoomed-in view of the active sites of human thrombin (3B9F34 ) and APC (from 1AUT), with modeled KRK sequence corresponding to the P2, P1, and P1′ positions found in the crystal structure of the PCI-thrombin complex.34
Active site properties of thrombin and APC. (A) Electrostatic surface representations of human thrombin (1PPB42 ) and human APC (from 1AUT44 ), with the small molecule inhibitor PPACK bound into the active site cleft (sticks). (B) Zoomed-in view of the active sites of human thrombin (3B9F34 ) and APC (from 1AUT), with modeled KRK sequence corresponding to the P2, P1, and P1′ positions found in the crystal structure of the PCI-thrombin complex.34
Specificity of PCI RCL Lys variants
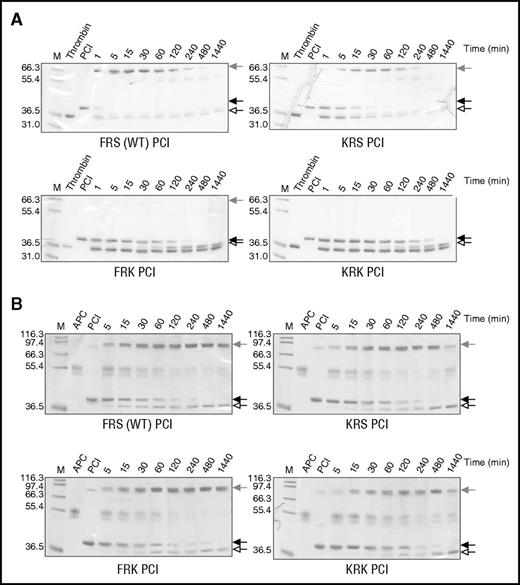
Individual and combined P2 and P1′ lysine variants were constructed on the PCI scaffold as described in “Methods.” The RCL of wt PCI has a P2 Phe, P1 Arg, and P1′ Ser, so we refer to wt as FRS. Separate and combined P2 and P1′ Lys variants are referred to as KRS, FRK, and KRK. To evaluate the effect of the mutations on the inhibitory activity of PCI, variants were incubated with either thrombin (Figure 2A) or APC (Figure 2B), and the reaction products were run on SDS-PAGE (Figure 2; supplemental Figure 3). This method allows simultaneous evaluation of both inhibitory and substrate behavior (supplemental Figure 2). We noted that wt PCI rapidly formed complex with thrombin with complete inhibition in about 1 minute. APC inhibition was considerably slower, with the reaction completed after about 4 hours. The P2 Lys (KRS) mutation slowed complex formation with thrombin, requiring 30 minutes to achieve full inhibition. The P1′ Lys mutation (FRK) essentially abolished thrombin inhibition, although the PCI was consumed as a substrate. The inhibitory profiles of these variants for APC were essentially identical to wt PCI, indicating that the substitutions were tolerated but did not improve APC recognition. The combined P2 and P1′ Lys mutations (KRK) resulted in complete loss of activity against thrombin, similar to what was found for the P1′ variant, but they appeared to slow inhibition of APC, approximately doubling the time require to achieve full inhibition relative to wt. Second-order rate constants for the inhibition of thrombin and APC by the PCI variants were determined (Table 1), and they agree with the qualitative SDS-PAGE results. Both the FRK and KRK variants of PCI exhibited a substantial increase in specificity for APC over thrombin (1600- and 400-fold, respectively); however, the rates of APC inhibition remained low.
Inhibition of thrombin and APC by PCI variants. Nonreducing SDS-PAGE of the reactions of (A) human plasma thrombin and (B) APC with PCI variants (FRS is wt, KRS is P2 Lys, FRK is P1′ Lys, and KRK is the combined P2 and P1′ Lys variant). Native serpin is indicated by a solid arrow, cleaved serpin by an open arrow, and the covalent serpin:protease complex by a shaded arrow. In each gel, lane 1 (M) is molecular weight markers, lane 2 is the protease alone, lane 3 is serpin alone, and the other lanes are the reactions of serpin and protease at the indicated times.
Inhibition of thrombin and APC by PCI variants. Nonreducing SDS-PAGE of the reactions of (A) human plasma thrombin and (B) APC with PCI variants (FRS is wt, KRS is P2 Lys, FRK is P1′ Lys, and KRK is the combined P2 and P1′ Lys variant). Native serpin is indicated by a solid arrow, cleaved serpin by an open arrow, and the covalent serpin:protease complex by a shaded arrow. In each gel, lane 1 (M) is molecular weight markers, lane 2 is the protease alone, lane 3 is serpin alone, and the other lanes are the reactions of serpin and protease at the indicated times.
Second-order rate constants of inhibition of thrombin and APC by PCI variants
| Variant . | k2 (M−1⋅s−1) . | SR . | |
|---|---|---|---|
| Thrombin . | APC . | ||
| wt PCI | (2.82 ± 0.15) × 104 | (6.80 ± 0.32) × 102 | 0.024 |
| KRS PCI* | 1.18 × 103* | 6.0 × 102* | 0.5* |
| FRK PCI | (2.24 ± 0.24) × 101 | (8.84 ± 0.74) × 102 | 39.5 |
| KRK PCI | (2.80 ± 0.24) × 101 | (2.80 ± 0.13) × 102 | 10 |
| Variant . | k2 (M−1⋅s−1) . | SR . | |
|---|---|---|---|
| Thrombin . | APC . | ||
| wt PCI | (2.82 ± 0.15) × 104 | (6.80 ± 0.32) × 102 | 0.024 |
| KRS PCI* | 1.18 × 103* | 6.0 × 102* | 0.5* |
| FRK PCI | (2.24 ± 0.24) × 101 | (8.84 ± 0.74) × 102 | 39.5 |
| KRK PCI | (2.80 ± 0.24) × 101 | (2.80 ± 0.13) × 102 | 10 |
Values are rate constants plus or minus standard error.
The rate determination for KRS PCI is a preliminary determination from only 2 measurements (therefore, no error is shown) and was calculated by dividing kobs by the serpin concentration. SR stands for specificity ratio of rate constant for APC over thrombin.
Specificity of α1AT RCL Lys variants
α1AT is the most prevalent serpin in the blood, circulating at a concentration of ∼2 g/L.38,39 It has a Met at the P1 position and therefore targets chymotrypsin-type serine proteases, including neutrophil elastase and cathepsin G. The Pittsburgh mutation, P1 Met-to-Arg, has been observed in a few patients who generally experience severe bleeding as a result of the switching of specificity toward the trypsin-type serine proteases, including the clotting factors thrombin and fXa.40,41 However, α1AT Pittsburgh is also a potent inhibitor of APC, with a rate constant 100 times that of PCI.30 We therefore hypothesized that α1AT Pittsburgh might serve as a better template for creating an APC-specific serpin using the Lys mutations identified from our work with PCI.
Lysine mutations were made individually and in combination at P2 and P1′ in α1AT Pittsburgh (α1AT has a Pro at the P2 position and a Ser at the P1′ position; PRS). Rates of inhibition of APC, thrombin, fXa, and fXIa were measured, and the results are given in Table 2. As seen with PCI, each single Lys mutation dramatically reduced inhibition of thrombin (∼5800-fold for KRS α1AT and ∼1700-fold for PRK α1AT), and the double mutation abolished thrombin inhibition. Inhibition of fXa, although still detectable, was reduced by 400-fold compared with only ∼10-fold for the single Lys mutants. Inhibition of fXIa was reduced by about 1000-fold for the double mutant. Crucially, APC inhibition was reduced by only sevenfold. The resultant increase in specificity ratio of KRK α1AT relative to PRS α1AT for APC over thrombin, fXa, and fXIa is >8000, 50, and 120 times, respectively.
Second-order rate constants of inhibition of thrombin, APC, fXa, and fXIa by α1AT variants
| Variant . | k2 (M−1·s−1) . | |||
|---|---|---|---|---|
| Thrombin . | APC . | fXa . | fXIa . | |
| PRS α1AT | (2.93 ± 0.18) × 105 | (1.08 ± 0.071) × 105 | (4.13 ± 0.24) × 104 | (4.00 ± 0.13) × 105 |
| KRS α1AT | (5.10 ± 0.28) × 101 | (6.48 ± 0.71) × 104 | (3.93 ± 0.31) × 103 | ND |
| PRK α1AT | (1.74 ± 0.17) × 102 | (9.57 ± 1.37) × 104 | (4.89 ± 0.16) × 103 | ND |
| KRK α1AT | No detectable inhibition | (1.51 ± 0.17) × 104 | (1.16 ± 0.10) × 102 | (4.71 ± 0.37) × 102 |
| Variant . | k2 (M−1·s−1) . | |||
|---|---|---|---|---|
| Thrombin . | APC . | fXa . | fXIa . | |
| PRS α1AT | (2.93 ± 0.18) × 105 | (1.08 ± 0.071) × 105 | (4.13 ± 0.24) × 104 | (4.00 ± 0.13) × 105 |
| KRS α1AT | (5.10 ± 0.28) × 101 | (6.48 ± 0.71) × 104 | (3.93 ± 0.31) × 103 | ND |
| PRK α1AT | (1.74 ± 0.17) × 102 | (9.57 ± 1.37) × 104 | (4.89 ± 0.16) × 103 | ND |
| KRK α1AT | No detectable inhibition | (1.51 ± 0.17) × 104 | (1.16 ± 0.10) × 102 | (4.71 ± 0.37) × 102 |
Values are rate constants plus or minus standard error.
ND, not determined.
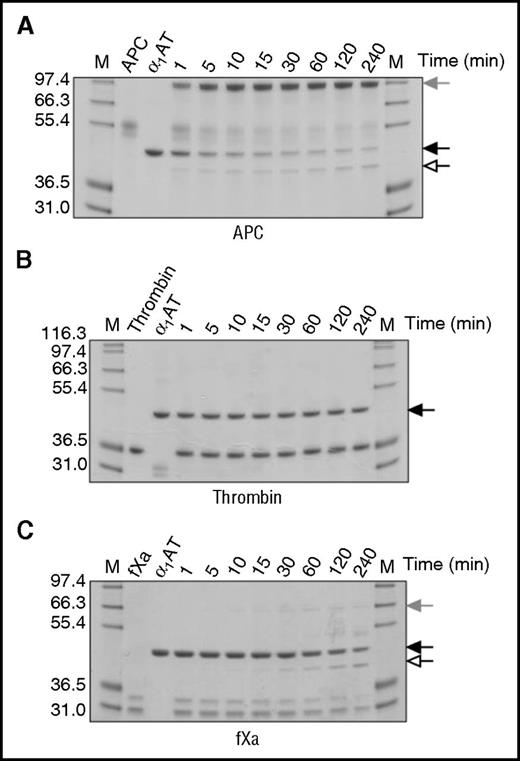
To determine whether the observed decrease in rate of procoagulant protease inhibition was the result of increased substrate behavior, reactions were visualized by SDS-PAGE (Figure 3). For these experiments, fIXa and thrombin:TM were also included (supplemental Figure 4). KRK α1AT rapidly formed a covalent complex with APC (Figure 3A), but no complex formation or cleavage was observed with thrombin, even after 4 hours (Figure 3B). Small amounts of complex were observed with fXa (Figure 3C) and fXIa (supplemental Figure 4F), consistent with the results from the kinetic studies. For fIXa, only a hint of inhibitory complex was observed (supplemental Figure 4E). The addition of TM to thrombin had no effect on the ability of thrombin to react with KRK α1AT (supplemental Figure 4D). These results indicate that KRK α1AT has a better inhibitory profile than KRK PCI and justify its selection as the candidate anti-APC serpin for efficacy studies.
Inhibition of proteases by KRK α1AT. SDS-PAGE of the reactions of (A) APC, (B) thrombin, and (C) fXa with KRK α1AT. Native serpin is indicated by a solid arrow, cleaved serpin by an open arrow, and the covalent serpin:protease complex by a shaded arrow. (A) and (B) are run under nonreducing conditions, and (C) is run under reducing conditions. In each gel, lane M shows molecular weight markers, lane 2 is the protease alone, lane 3 is KRK α1AT alone, and the other lanes are the reactions at the indicated times.
Inhibition of proteases by KRK α1AT. SDS-PAGE of the reactions of (A) APC, (B) thrombin, and (C) fXa with KRK α1AT. Native serpin is indicated by a solid arrow, cleaved serpin by an open arrow, and the covalent serpin:protease complex by a shaded arrow. (A) and (B) are run under nonreducing conditions, and (C) is run under reducing conditions. In each gel, lane M shows molecular weight markers, lane 2 is the protease alone, lane 3 is KRK α1AT alone, and the other lanes are the reactions at the indicated times.
Effect of KRK α1AT on clotting times and thrombin generation in vitro
For KRK α1AT to be a useful hemostatic agent, it must be devoid of any residual anticoagulant activity. We therefore spiked normal pooled plasma with PRS (control) or KRK α1AT and conducted PT and aPTT assays (Table 3). The PRS α1AT control increased clotting times in both assays; however, addition of KRK α1AT to normal plasma had no effect on PT and only a marginal effect on aPTT, consistent with some residual activity against fXIa (Table 2). The residual anti-fXa activity of KRK α1AT appears to be too low (116 M–1s−1; Table 2) to affect clotting times in these assays. We concluded that KRK α1AT does not possess any relevant anticoagulant activity.
Clotting times for PT and aPTT assays with PRS and KRK α1AT (5 μM)
| Variant . | Clotting time (s) . | |
|---|---|---|
| PT . | aPTT . | |
| Buffer | 16.6 ± 0.22 | 55.0 ± 3.8 |
| PRS α1AT | 50.5 ± 5.6 | >300 |
| KRK α1AT | 16.5 ± 0.3 | 62.1 ± 4.2 |
| Variant . | Clotting time (s) . | |
|---|---|---|
| PT . | aPTT . | |
| Buffer | 16.6 ± 0.22 | 55.0 ± 3.8 |
| PRS α1AT | 50.5 ± 5.6 | >300 |
| KRK α1AT | 16.5 ± 0.3 | 62.1 ± 4.2 |
The values shown are an average of 3 measurements with standard deviation.
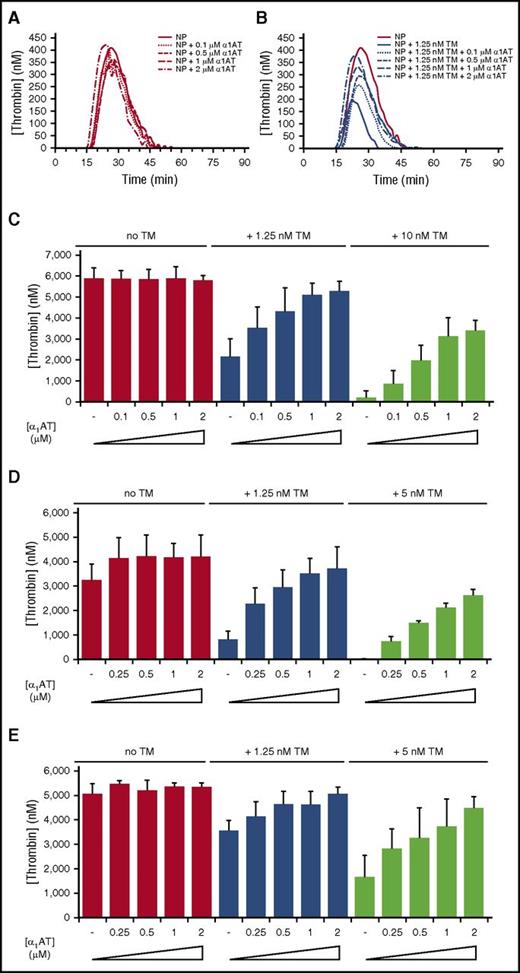
To assess the procoagulant activity of KRK α1AT, we used a TGA in the absence and presence of TM with PRS α1AT as control (Figure 4; supplemental Figure 5). Two concentrations of TM were chosen empirically: one that resulted in an ∼50% reduction in thrombin generation and one that essentially abolished thrombin generation. The control anticoagulant PRS α1AT completely inhibited thrombin generation in normal plasma when added at a concentration of 0.5 µM (supplemental Figure 5A). Consistent with results from the PT assay, KRK α1AT had no effect on thrombin generation when added to normal pooled plasma (Figure 4A). However, when TM was added, KRK α1AT dose-dependently increased thrombin generation (Figure 4B-C). Similar results were obtained in a TGA using plasma from patients with hemophilia A or hemophilia B (Figure 4D-E; supplemental Figures 6 and 7).
KRK α1AT rescues the effect of soluble TM in normal and factor-deficient human plasma. (A-B) Representative thrombin generation curves showing the effect of KRK α1AT (concentrations indicated) in normal human plasma (NP) in the (A) absence and (B) presence of 1.25 nM soluble TM. Each curve is an average of duplicates. In (B) the no TM control from (A) is shown for reference. (C) Bar graph of total thrombin generation in normal plasma (given as thrombin concentrations) at 0, 1.25, and 10 nM TM, with increasing concentrations of KRK α1AT (up to 2 μM). Each bar is the average of 3 experiments of duplicates, with standard deviation shown by vertical lines. (D) Bar graph of total thrombin generation in fVIII-deficient (hemophilia A) plasma (given as thrombin concentrations) at 0, 1.25, and 5 nM TM, with increasing concentrations of KRK α1AT (up to 2 μM). Each bar is the average of 2 experiments of duplicates with standard deviation shown. (E) Bar graph of total thrombin generation in fIX-deficient (hemophilia B) plasma (given as thrombin concentrations) at 0, 1.25, and 5 nM TM, with increasing concentrations of KRK α1AT (up to 2 μM). Each bar is the average of at least 2 experiments of duplicates, with standard deviation shown.
KRK α1AT rescues the effect of soluble TM in normal and factor-deficient human plasma. (A-B) Representative thrombin generation curves showing the effect of KRK α1AT (concentrations indicated) in normal human plasma (NP) in the (A) absence and (B) presence of 1.25 nM soluble TM. Each curve is an average of duplicates. In (B) the no TM control from (A) is shown for reference. (C) Bar graph of total thrombin generation in normal plasma (given as thrombin concentrations) at 0, 1.25, and 10 nM TM, with increasing concentrations of KRK α1AT (up to 2 μM). Each bar is the average of 3 experiments of duplicates, with standard deviation shown by vertical lines. (D) Bar graph of total thrombin generation in fVIII-deficient (hemophilia A) plasma (given as thrombin concentrations) at 0, 1.25, and 5 nM TM, with increasing concentrations of KRK α1AT (up to 2 μM). Each bar is the average of 2 experiments of duplicates with standard deviation shown. (E) Bar graph of total thrombin generation in fIX-deficient (hemophilia B) plasma (given as thrombin concentrations) at 0, 1.25, and 5 nM TM, with increasing concentrations of KRK α1AT (up to 2 μM). Each bar is the average of at least 2 experiments of duplicates, with standard deviation shown.
Effect of KRK α1AT on clotting in vivo
The high sequence identities between human and mouse APC and thrombin (72% and 87%, respectively) suggest that the relevant features of the active sites are preserved. This was confirmed by comparing structures of human42 and mouse thrombin43 (supplemental Figure 8A) and by comparing a mouse APC model with the crystal structure of human APC44 (supplemental Figure 8B). Mouse thrombin did not react with KRK α1AT (supplemental Figure 9), and inhibition of mouse APC was preserved (twofold reduction in rate constant; supplemental Table 2). In addition, KRK α1AT dose-dependently increased thrombin generation in mouse hemophilia B plasma spiked with TM (supplemental Figure 10). We concluded that mouse models of hemostasis could be used to test the in vivo efficacy of KRK α1AT.
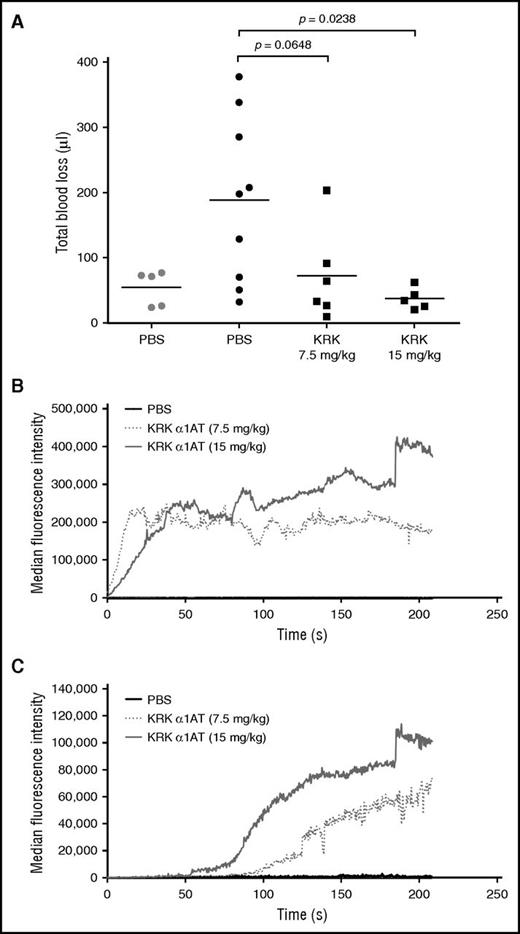
Two assays were used to assess the ability of KRK α1AT to promote hemostasis in a hemophilia B mouse: tail clip and intravital laser injury. In the tail clip assay, the tail was transected, and blood was collected for 10 minutes after administration of either KRK α1AT or PBS, and total blood loss volume was calculated from a standard hemoglobin curve after red cell lysis.35 In this model, KRK α1AT dose-dependently decreased the blood loss (Figure 5A), with the highest dose (15 mg/kg) reducing bleeding to the same level as for wt mice. The effect of KRK α1AT was also assessed by using the cremaster arteriole laser injury model.45 Clot formation was visualized by fluorescence microscopy following deposition of platelets and fibrin with time. KRK α1AT dose-dependently increased in the amount of platelet (Figure 5B) and fibrin deposition (Figure 5C). Example images taken as snapshots from representative recorded videos are provided in supplemental Figure 11. At the 7.5 mg/kg dose of KRK α1AT, stable clots (platelets and fibrin) occurred in 55% of injuries compared with 0% when only PBS was given. For the higher dose (15 mg/kg), stable clots were observed in 85% of injuries (Table 4). Fluorescence quantitation showed a shorter lag time for fibrin deposition at the higher dose (Figure 5C).
KRK α1AT reduces bleeding and increases platelet and fibrin deposition in hemophilia B mice. (A) Total blood loss of either wt (gray) or hemophilia B (black) mice injected with either PBS (circles), or KRK α1AT (squares) after tail clip. Each data point is from a single mouse. Lines show the average per group. P values were calculated by using an unpaired Student t test. (B-C) Median accumulation of (B) platelets and (C) fibrin over time after laser-induced arteriole injury after administration of PBS (black line; 8 total thrombi, 5 mice) or KRK α1AT (gray-shaded dotted and solid lines; 18 total thrombi, 4 mice at 7.5 mg/kg; 20 total thrombi, 3 mice at 15 mg/kg).
KRK α1AT reduces bleeding and increases platelet and fibrin deposition in hemophilia B mice. (A) Total blood loss of either wt (gray) or hemophilia B (black) mice injected with either PBS (circles), or KRK α1AT (squares) after tail clip. Each data point is from a single mouse. Lines show the average per group. P values were calculated by using an unpaired Student t test. (B-C) Median accumulation of (B) platelets and (C) fibrin over time after laser-induced arteriole injury after administration of PBS (black line; 8 total thrombi, 5 mice) or KRK α1AT (gray-shaded dotted and solid lines; 18 total thrombi, 4 mice at 7.5 mg/kg; 20 total thrombi, 3 mice at 15 mg/kg).
Platelet and fibrin deposition in a cremaster arteriole laser-induced injury model
| Sample . | No. of mice . | Total injuries . | Clot formation . | |||||
|---|---|---|---|---|---|---|---|---|
| No clot . | Platelets only . | Platelets + fibrin . | ||||||
| No. . | % . | No. . | % . | No. . | % . | |||
| PBS | 5 | 8 | 8 | 100 | 0 | 0 | 0 | 0 |
| KRK α1AT (7.5 mg/kg) | 4 | 18 | 2 | 11.1 | 6 | 33.3 | 10 | 55.6 |
| KRK α1AT (15 mg/kg) | 3 | 20 | 0 | 0 | 3 | 15 | 17 | 85 |
| Sample . | No. of mice . | Total injuries . | Clot formation . | |||||
|---|---|---|---|---|---|---|---|---|
| No clot . | Platelets only . | Platelets + fibrin . | ||||||
| No. . | % . | No. . | % . | No. . | % . | |||
| PBS | 5 | 8 | 8 | 100 | 0 | 0 | 0 | 0 |
| KRK α1AT (7.5 mg/kg) | 4 | 18 | 2 | 11.1 | 6 | 33.3 | 10 | 55.6 |
| KRK α1AT (15 mg/kg) | 3 | 20 | 0 | 0 | 3 | 15 | 17 | 85 |
Discussion
This study was motivated by the observation that bleeding frequency and severity are reduced in patients with hemophilia A who co-inherit the fV Leiden mutation.25-27 These observations were supported by crossing hemophilia A and hemophilia B mice with fV Leiden mice and conducting tail clip and intravital microscopy studies.46 We thus hypothesized that a serpin with specificity for APC might be an effective hemostatic agent.
The mechanism of all hemophilia treatments is to increase the amount of thrombin generated at sites of vascular damage. This is generally achieved by adding missing factors or by boosting the concentration of either the enzymes or substrates that lead to the production of thrombin. The fV Leiden mutation and, to a potentially greater extent, direct APC inhibition increases thrombin generation by allowing already-formed prothrombinase more time to function.
Our goal was to engineer a serpin that would inhibit APC with a rate constant of greater than 1 × 104 M–1s−1, but without appreciable inhibition of any relevant procoagulant protease. On the basis of differences in features of the active sites of thrombin and APC, we chose to alter the 2 residues flanking the primary specificity-determining P1 residue (P2 and P1′). Mutating these to Lys in PCI had the desired effect on selectivity, but the rate of inhibition of APC was unacceptably low. Although the rate of inhibition was increased by adding heparin (data not shown), the relevance of glycosaminoglycans in mediating APC inhibition by PCI in vivo is unclear.
We therefore decided to change the serpin scaffold to α1AT Pittsburgh, which is known to rapidly inhibit APC and procoagulant proteases. As with the PCI template, the KRK mutations in the RCL of α1AT abrogated inhibition of the tested coagulation proteases while maintaining an ability to rapidly inhibit APC. Importantly, coagulation was not inhibited by the addition of KRK α1AT to normal or hemophilia plasma, even at concentrations of 5 μM. KRK α1AT did, however, retain some residual inhibitory activity against fXIa reflected in slightly prolonged aPTTs. However, the main function of fXIa in coagulation is the activation of fIX to support formation of the intrinsic Xase complex. Deficiency in either fVIII or fIX, the basis of hemophilia, therefore renders fXIa redundant.
It is important to note that the studies described here used protein purified from an E coli expression system, perhaps explaining the relatively high doses required for efficacy in the mouse models. Although the activity of α1AT is not affected by glycosylation,47 its absence will reduce the half-life of the protein.48 Therefore, the actual exposure of KRK α1AT in the efficacy experiments is likely to be substantially lower than what would be expected on the basis of dose. PK and efficacy studies using glycosylated KRK α1AT will need to be conducted in the future to determine the actual relationship between dose and efficacy.
One major advantage of choosing α1AT as the scaffold anti-APC serpin is its 5- to 7-day half-life in the circulation.49 In addition, in contrast to current bypassing agents, which are administered intravenously, the subcutaneous route of administration for KRK α1AT might also be available. Studies in rabbits showed that the pharmacodynamics of subcutaneously administered plasma human α1AT were comparable to intravenously administrated protein.50 The risk for cross-reacting antibodies developing in response to KRK α1AT is also likely to be low because wt α1AT is present at a high concentration (∼2 g/L) in plasma,38,39 and immune tolerance to a protein is strongly related to its endogenous level.51,52 Therefore, choosing α1AT as our scaffold for developing an APC-specific serpin confers several possible attractive features: fast and specific APC inhibition, long half-life, possible subcutaneous route of administration, and low immunogenic potential. If the half-life and subcutaneous availability of KRK α1AT is similar to what has been reported for wt α1AT, it is possible that weekly or fortnightly subcutaneous injections could provide effective prophylaxis for either form of hemophilia, even when inhibitors are present.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Wellcome Trust studentship (S.G.I.P.).
Authorship
Contribution: J.A.H., T.E.A., T.P.B., and S.G.I.P. conceived of and designed the study; S.G.I.P. conducted all in vitro experiments; L.I., R.M.C., and S.G.I.P. designed and L.I. conducted the in vivo experiments; and J.A.H. and S.G.I.P. wrote the manuscript.
Conflict-of-interest disclosure: J.A.H., T.P.B., and S.G.I.P. have shares in ApcinteX Ltd (a company founded to develop KRK α1AT). J.A.H., T.P.B., S.G.I.P., and T.E.A. participate in revenue sharing through the commercialization arm of the University of Cambridge. The results discussed in this manuscript form part of patent application, Modified Serpins for the Treatment of Bleeding Disorders (WO 2015086854 A1). The remaining authors declare no competing financial interests.
Correspondence: James A. Huntington, University of Cambridge, Cambridge Institute for Medical Research, Wellcome Trust/MRC Building, Hills Rd, Cambridge CB2 0XY, United Kingdom; e-mail: jah52@cam.ac.uk.