In this issue of Blood, Polderdijk et al design and evaluate a therapeutic inhibitor of activated protein C.1 They have produced a recombinant variant of α1-antitrypsin (α1AT) incorporating 3 residue changes within the P2-P1′ sequence of its reactive loop. This variant (termed KRK α1AT) exhibits high specificity and inhibitory efficiency toward activated protein C. It is able to restore thrombin generation in normal and in hemophilia plasmas, when these are supplemented with soluble thrombomodulin. It is able to restore hemostasis in challenged hemophilia mice. KRK α1AT is therefore a potentially valuable future therapeutic agent for the human hemophilias.
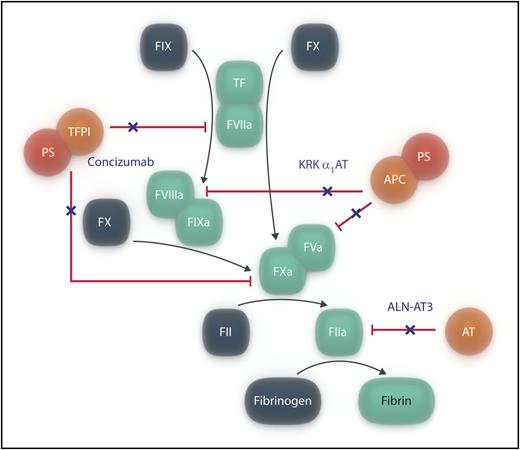
Simplified representation of blood coagulation, indicating the 3 anticoagulant pathways (in orange/red). Agents that have recently been shown to target the anticoagulant pathways and that are proposed as potential therapies for hemophilia are indicated in dark blue text, and their actions are shown by a dark blue “×.” For simplicity, only thrombin is shown to be inhibited by antithrombin. APC, activated protein C; AT, antithrombin; PS, protein S; TFPI, tissue pathway inhibitor. Professional illustration by Somersault18:24.
Simplified representation of blood coagulation, indicating the 3 anticoagulant pathways (in orange/red). Agents that have recently been shown to target the anticoagulant pathways and that are proposed as potential therapies for hemophilia are indicated in dark blue text, and their actions are shown by a dark blue “×.” For simplicity, only thrombin is shown to be inhibited by antithrombin. APC, activated protein C; AT, antithrombin; PS, protein S; TFPI, tissue pathway inhibitor. Professional illustration by Somersault18:24.
The initial phase of blood coagulation is triggered by tissue factor (TF) exposed on damage to the vascular endothelium, making contact with factor VII(a) [FVII(a)] circulating in the blood. The TF/FVIIa complex rapidly activates FX, enabling trace amounts of thrombin to be generated from prothrombin. Thrombin that is formed rapidly activates FVIII and FV, enabling them to form their respective tenase and prothrombinase complexes required for an effective propagation phase of coagulation.
Three important anticoagulant pathways control coagulation (see figure). The initiation step is regulated by the Kunitz-type inhibitor,2 TFPI, which forms an inactive complex with FXa and TF/FVIIa: protein S acts as a cofactor to TFPI. Second, antithrombin is an important direct inhibitor of thrombin and other coagulation proteases.3 Finally, the propagation phase is regulated by the protein C anticoagulant pathway.4 As thrombin is generated, it binds to endothelial cell surface thrombomodulin, and its specificity is redirected from fibrinogen cleavage to protein C activation. Activated protein C, with its essential cofactor protein S, is able to inactivate FVa (and possibly FVIIIa), thereby down-regulating any burst of thrombin generation.
The bleeding disorders, hemophilia A and B, are caused by inherited deficiencies of FVIII and FIX, respectively. They can be viewed as arising from functional defects of the propagation phase of coagulation. An attractive concept for understanding the range of hemostatic disorders, encompassing at extremes the inherited bleeding and the thrombotic disorders, has been that of hemostatic balance/imbalance. Recent reports have suggested the value of reducing the functions of anticoagulant pathways to correct the deficiency in procoagulant pathways that causes hemophilia. Experimental studies of administration of monoclonal antibodies to TFPI5,6 have demonstrated improved hemostasis in animal models of hemophilia. A phase 1 trial of administration of 1 of these (concizumab)7 has shown increased thrombin generation both in normal subjects and in patients with hemophilia. The antithrombin anticoagulant pathway has been suppressed by administration of a RNA interference agent (ALN-AT3) in mice and in nonhuman primates8 : hemostasis in mouse models of hemophilia was improved. A phase 1 study of this agent in normal patients and in patients with hemophilia is currently underway.9 These investigations collectively suggest a poised equilibrium between procoagulant and anticoagulant pathways.
Whether inhibition of the protein C anticoagulant pathway might also provide opportunities for redressing the hemostatic imbalance in hemophilia has been uncertain, but has now been investigated by Polderdijk et al. There are interesting issues surrounding this approach. It has not been known whether appreciable thrombin can be generated in hemophilia. If thrombin is not generated, targeting an active protease (activated protein C) produced as a consequence of its action might be ineffective. Second, the way in which activated protein C is regulated by natural inhibitors remains uncertain, as no single inhibitor has high specificity for this protease. Third, any novel therapeutic approach for targeting activated protein C in the context of hemophilia would best use an agent with long half-life, as patients with hemophilia would not welcome treatments requiring additional frequent (IV) injections: low molecular weight chemical inhibitors might only be favored if they could be taken orally. Polderdijk et al first explored the inhibitory activities and specificities of variant forms of the serpin, protein C inhibitor (PCI), using a systematic mutation strategy and expression of the variants in a bacterial expression system. PCI is a known weak inhibitor of activated protein C, but also of thrombin. Reactive loop variants were generated by Polderdijk et al that had greatly increased specificity for activated protein C. However, their absolute inhibitory rates against this protease remained poor.
They were more successful when they prepared variants of the serpin, α1AT. A mutant form of this inhibitor, α1AT Pittsburgh, is a known potent inhibitor of serine proteases such as thrombin. The triple-residue mutant, KRK α1AT, was found to be a highly selective and potent inhibitor of activated protein C. Although KRK α1AT had no activity against thrombin, it did express low (<100-fold) inhibitory activity against both FXa and FXIa. Reassuringly, KRK α1AT had no effect on thrombin generation in normal human plasma in the absence of soluble thrombomodulin, but in its presence, KRK α1AT was able to inhibit the induced anticoagulant effect by activated protein C. Critically, Polderdijk et al were able to restore/modulate hemostatic balance with KRK α1AT in bleeding and thrombotic models, using hemophilic mice.
Polderdijk et al suggest important advantages of using KRK α1AT as a therapeutic agent. Its subcutaneous administration and long half-life are very attractive features, particularly if its activity is maintained when expressed in cells that can add half-life-prolonging glycan chains. Off-target effects and any induced immune response to the variant inhibitor must, of course, remain minimal. One potential issue concerning the therapeutic administration of inhibitors of activated protein C is the possible inhibition of its anti-inflammatory properties, mediated through PAR activation of the endothelium. Current investigations of nonanticoagulant activated protein C (with a variant that retains anti-inflammatory action) in human stroke are directed at enhancing this pathway to provide neuroprotection.10 Prolonged inhibition of activated protein C in patients with hemophilia with KRK α1AT may reduce cytoprotective endothelial cell PAR activation, the consequences of which remain to be determined.
Conflict-of-interest disclosure: The author has acted as an expert witness for a company, Novo Nordisk, with a product in development (concizumab) mentioned in this article.