Key Points
CD19− relapses are a major challenge in about 10% to 20% of patients treated with blinatumomab.
Molecular workup of 1 case revealed a disrupted CD19 membrane export as the basis for blinatumomab resistance.
Abstract
The CD19 antigen is a promising target for immunotherapy of acute lymphoblastic leukemia (ALL), but CD19− relapses remain a major challenge in about 10% to 20% of patients. Here, we analyzed 4 CD19− ALL relapses after treatment with the CD19/CD3 bispecific T-cell engager (BiTE) blinatumomab. Three were on-drug relapses, with the CD19− escape variant first detected after only 2 treatment courses. In 1 patient, the CD19− clone appeared as a late relapse 19 months after completion of blinatumomab treatment. All 4 cases showed a cellular phenotype identical to the primary diagnosis except for CD19 negativity. This argued strongly in favor of an isolated molecular event and against a common lymphoid CD19− progenitor cell or myeloid lineage shift driving resistance. A thorough molecular workup of 1 of the cases with early relapse confirmed this hypothesis by revealing a disrupted CD19 membrane export in the post–endoplasmic reticulum compartment as molecular basis for blinatumomab resistance.
Introduction
CD19, a B-lineage–specific molecule, is a promising target in the treatment of hematological B-lineage cancers such as B-lineage acute lymphoblastic leukemias (ALL). The receptor functions as a costimulatory molecule of the B-cell receptor along with CD21 and CD81. The most promising CD19-targeting immunotherapies to date comprise CD19-directed chimeric antigen receptor (CAR) T cells (CART-19) and blinatumomab, a bispecific anti-CD19/CD3 T-cell engaging antibody (bispecific T-cell engager [BiTE]). Both concepts have shown outstanding effects in ALL.1-6 However, relapse with CD19− leukemic blasts remains a challenge in ∼10% to 20% of patients treated with either CART-19 or BiTEs.3,7-9 Also, in patients after chemotherapy or transplantation, diminished CD19 expression (but not loss of CD19) has been previously described on relapse blasts,10 also confirming the notion that this antigen is not essential for leukemia maintenance.11
The mechanisms underlying the emergence of CD19− escape variants are insufficiently understood to date but should be elucidated because this may have implications for the optimization of CD19-directed immunotherapies. In patients treated with CART-19, genetic alterations and alternatively spliced, truncated CD19 variants have been described to account for resistance.12 In addition, a myeloid lineage shift has been shown to drive resistance in patients with mixed lineage leukemia–rearranged ALL on blinatumomab and CAR–T-cell therapy.13,14
Here, we report on the clinical and laboratory characteristics of a small series of CD19− relapses in blinatumomab-treated ALL patients. In 1 exemplary case, we go on to show how disruption of CD19 trafficking as a novel mechanism of resistance may account for loss of CD19 surface positivity.
Study design
See supplemental Methods (available on the Blood Web site) for further details.
Flow cytometry
Routine diagnostic workup was performed for patients 1 to 4 at initial diagnosis, at the time point of first relapse/refractory disease (if applicable) and during the course of blinatumomab treatment. CD81 stainings were performed in patient 3. Minimal concentrations of anti-CD19 clones HIB19 and J3-119 saturating CD19-binding sites were used for competition assays with Nalm-6 cells preincubated with increasing concentrations of blinatumomab.
Sequencing of CD19 and CD81 exons and mRNA detection
All CD19 and CD81 exons were subjected to next-generation sequencing (NGS) on an Illumina MiSeq platform. Messenger RNA (mRNA) was isolated, and transcribed into complementary DNA, CD19, and CD81 transcripts amplified and visualized on agarose gels.
Western blot analyses
ALL cells from patient 3 were protein extracted and subjected to western blot analysis using a CD19 antibody as well as different lectins for global glycosylation analysis (phaseolus vulgaris erythroagglutinin [PHA-E], concanavalin A [Con A], wheat germ agglutinin [WGA], maackia amurensis lectin [MAL]). Chronic lymphocytic leukemia (CLL) (± digestion with peptide-N-glycosidase F) and healthy donor cells were used as controls.
Results and discussion
Patient characteristics
Patient 1 presented with persistence of blasts after second consolidation and was then started on blinatumomab (trial NCT01466179). He achieved a hematological complete response (CR) after the first blinatumomab course but developed a CD19− clone after the second course.
Patient 2 developed relapse after high-dose methotrexate/asparaginase in week 30. The patient was recruited for experimental treatment with blinatumomab (trial NCT01466179). Hematological CR after the first course of blinatumomab was quickly followed by a CD19− relapse after the second course.
Patient 3 (with mixed lineage leukemia–rearranged ALL) achieved hematologic CR after second consolidation. Minimal residual disease (MRD) positivity triggered treatment with blinatumomab (trial NCT00560794), but at the time point of blinatumomab initiation the patient had already developed a full-blown CD19+ relapse. CD19− relapse occurred after the second course of blinatumomab.
Patient 4 relapsed at first reinduction. She received 5 courses of blinatumomab and achieved MRD− remission (trial NCT01466179). Late CD19− relapse occurred 19 months after discontinuation of blinatumomab.
Patient characteristics are summarized in Table 1.
Patient characteristics and ALL phenotypes
| No. . | Age, y/Sex . | ALL subtype (genetics) . | Response to primary treatment . | Treatment of relapsed/ refractory disease or MRD positivity . | Emergence of CD19− clone (flow cytometry)/ hematological relapse . | Initial phenotype (before blinatumomab) . | Phenotype at CD19− relapse (after blinatumomab) . |
|---|---|---|---|---|---|---|---|
| 1 | 60/M | Pre B-ALL | GMALL Elderly 1/2003: Persistence of CD19+ blasts after second consolidation (week 24) | Blinatumomab phase 2 study (NCT01466179) | After second blinatumomab course/ after third blinatumomab course | CD19+/CD34+/CD10+/ CD45(+)/cyTdT+/ cyCD79a+/cyCD22+/ cyIgM+ | CD19−/otherwise identical to initial phenotype |
| 2 | 30/F | c-ALL | GMALL 7/2003: Relapse with CD19+ blasts at second reinduction (week 30) | Blinatumomab phase 2 study (NCT01466179) | After second blinatumomab course/ after third blinatumomab course | CD19+/CD34+/CD10+/ CD45(+)/HLA-DR+/ cyTdT+/cyCD79a+ | CD19−/otherwise identical to initial phenotype |
| 3 | 65/M | Pro B-ALL (t(11;19)) | GMALL Elderly 1/2003: MRD+ after second consolidation (week 24) | Blinatumomab phase 2 study (NCT00560794) | After second blinatumomab course/ after second blinatumomab course | CD19+/CD34+/ CD10−/CD45(+)/ HLA-DR(+)/cyTdT+ | CD19−/otherwise identical to initial phenotype |
| 4 | 74/F | c-ALL (trisomy 9, monosomy 7 and 3, 6q21+, 6q23+) | GMALL Elderly 1/2003: Relapse with CD19+ blasts at first reinduction (week 24) | Blinatumomab phase 2 study (NCT01466179) | 19 mo after completion of blinatumomab/ 19 mo after completion of blinatumomab | CD19+/CD34+/CD10(+)/ CD45(+)/cyTdT+/ CD20+ | CD19−/otherwise identical to initial phenotype |
| No. . | Age, y/Sex . | ALL subtype (genetics) . | Response to primary treatment . | Treatment of relapsed/ refractory disease or MRD positivity . | Emergence of CD19− clone (flow cytometry)/ hematological relapse . | Initial phenotype (before blinatumomab) . | Phenotype at CD19− relapse (after blinatumomab) . |
|---|---|---|---|---|---|---|---|
| 1 | 60/M | Pre B-ALL | GMALL Elderly 1/2003: Persistence of CD19+ blasts after second consolidation (week 24) | Blinatumomab phase 2 study (NCT01466179) | After second blinatumomab course/ after third blinatumomab course | CD19+/CD34+/CD10+/ CD45(+)/cyTdT+/ cyCD79a+/cyCD22+/ cyIgM+ | CD19−/otherwise identical to initial phenotype |
| 2 | 30/F | c-ALL | GMALL 7/2003: Relapse with CD19+ blasts at second reinduction (week 30) | Blinatumomab phase 2 study (NCT01466179) | After second blinatumomab course/ after third blinatumomab course | CD19+/CD34+/CD10+/ CD45(+)/HLA-DR+/ cyTdT+/cyCD79a+ | CD19−/otherwise identical to initial phenotype |
| 3 | 65/M | Pro B-ALL (t(11;19)) | GMALL Elderly 1/2003: MRD+ after second consolidation (week 24) | Blinatumomab phase 2 study (NCT00560794) | After second blinatumomab course/ after second blinatumomab course | CD19+/CD34+/ CD10−/CD45(+)/ HLA-DR(+)/cyTdT+ | CD19−/otherwise identical to initial phenotype |
| 4 | 74/F | c-ALL (trisomy 9, monosomy 7 and 3, 6q21+, 6q23+) | GMALL Elderly 1/2003: Relapse with CD19+ blasts at first reinduction (week 24) | Blinatumomab phase 2 study (NCT01466179) | 19 mo after completion of blinatumomab/ 19 mo after completion of blinatumomab | CD19+/CD34+/CD10(+)/ CD45(+)/cyTdT+/ CD20+ | CD19−/otherwise identical to initial phenotype |
c-ALL, common ALL; cy, cytoplasmic; F, female; GMALL, German multicenter study group for adult ALL; IgM, immunoglobulin M; M, male; TdT, terminal deoxynucleotidyl transferase.
Detection of CD19 with flow cytometry antibodies during blinatumomab treatment
Because CD19− relapse was diagnosed in 3 of 4 patients while on treatment with blinatumomab, competition assays on CD19+ NALM-6 cells were performed to find out if some of the commonly used flow cytometry detection antibodies may have competed with blinatumomab bound to CD19 and therefore may have falsely suggested CD19 negativity. Anti-CD19 clones HIB19 and J3-119 competed only with blinatumomab at concentrations largely exceeding the serum levels reached during continuous blinatumomab infusion (0.5-1 ng/mL15 ; supplemental Figures 1 and 2). These data suggest that serum blinatumomab does not interfere with detection of CD19 on routine flow cytometry.
Emergence and evolution of CD19− escape variants during blinatumomab treatment
Interestingly, in all subjects with on-drug emerging resistance (patients 1-3), the CD19− escape variant was first detected by flow cytometry after only 2 courses of blinatumomab. Cytological relapse followed no later than after 3 courses of blinatumomab (Table 1). These data should raise our awareness for imminent relapses that may be picked up very early by standard flow cytometry analysis.
Analysis of ALL primary and relapse immunophenotypes
Apart from CD19 loss, the flow cytometric ALL immunophenotypes were essentially unchanged compared with the initial phenotype in all patients (Table 1). Representative plots are shown in supplemental Figures 3 and 4. Interestingly, patient 4 (late relapse) showed a clearly regenerated CD19+ mature B-cell compartment (CD45+/CD20+/CD10−) along with her CD19− ALL blasts, suggesting lack of persistent immune-mediated anti-CD19 pressure at the time point of relapse. This is also compatible with the reported timing of immunoglobulin regeneration within 1 year after blinatumomab treatment initiation.16 This raises the possibility that the CD19 loss escape variant may have emerged early (during active CD19-directed immunological pressure) but remained dormant until clinical relapse. All other patients did not show evidence for CD19+ mature B cells which was expected due to ongoing blinatumomab treatment.
The preserved ALL phenotypes (except for CD19 negativity) clearly exclude a myeloid shift as resistance mechanism in these 4 patients. Moreover, they strongly argue against a more immature CD19− common lymphoid progenitor subpopulation that could have been present at initial diagnosis and could have evolved under the selective pressure of blinatumomab. Instead, our data support the notion that an ALL blast from the homogeneous bulk of ALL cells acquired CD19 loss as resistance-mediating property as a result of an isolated molecular event. The CD19− subpopulation may have emerged not only during treatment with blinatumomab, but also may have been present already at diagnosis or (predominantly) CD19+ relapse as a small, immunophenotypically invisible subpopulation. The latter hypothesis may even be more likely given the growing body of evidence from empiric studies and mathematical modeling in different tumor entities that minimal tumor subclones with resistance-mediating properties are present even before selective pressure is applied.17
Disrupted CD19 trafficking accounts for blinatumomab resistance in ALL patient 3
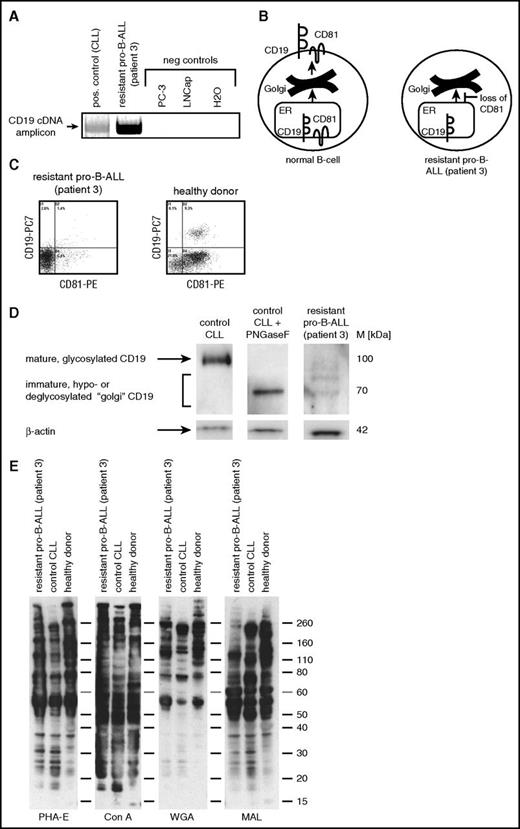
We performed a thorough molecular workup of 1 of the patients with available material and early CD19− relapse providing valuable insights into 1 of the mechanisms that may underlie resistance to CD19-directed immunotherapy. ALL blasts at relapse showed the same genetic lesion t(11;19) as at primary diagnosis and no structural abnormalities of chromosome 16 where the CD19 gene is located. CD19 expression was absent as demonstrated by flow cytometry with CD19 antibodies exhibiting differential epitope recognition (data not shown). This suggested a complete antigen-loss escape variant in contrast to a mere epitope loss or splice variant. Genomic NGS of CD19 revealed wild-type sequences of all 15 exons (data not shown). Because full-length CD19 mRNA was detectable in this patient, this suggested an evasive mechanism not based on transcriptional regulation and, again, argued against a CD19 splice variant (Figure 1A). Also, CD21, as another component of the CD19-associated complex, was not expressed in the ALL blasts of patient 3 (data not shown). We therefore searched for alterations in CD81, a protein that is involved in CD19/CD21/B-cell receptor complex function and that regulates CD19 protein maturation and trafficking as a chaperone from the Golgi to the cell surface (schematically shown in Figure 1B). Interestingly, leukemic blasts were negative for surface CD81 (Figure 1C). This was due to posttranscriptional regulation because targeted genomic NGS did not yield CD81 mutations (described as a mechanism in antibody deficiency syndrome18 ) or transcriptional silencing (data not shown). Further western blot analyses showed hypoglycosylated and deglycosylated, immature CD19 precursors in ALL patient 3, while fully glycosylated CD19 was absent (Figure 1D), and no general glycosylation deficiency was apparent in this patient as evidenced by lectin blots (Figure 1E). This further confirmed that lack of CD81 prevented CD19 processing and maturation in the Golgi.
CD81 loss in blasts from ALL patient 3 disrupts CD19 membrane trafficking. (A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of CD19 mRNA expression in ALL patient 3 and primary CLL+ control cells as well as PC-3 and LNCap prostate negative control cell lines. (B) Schematic representation of CD81-dependent CD19 cell surface transport via endoplasmic reticulum (ER) and Golgi compartments. (C) Flow cytometric analysis of CD81 membrane expression on blasts from ALL patient 3 and healthy donor CD19+ B cells. (D) CD19 western blot analysis using lysates from ALL patient 3 and control CLL cell lysates ± PNGaseF treatment of deglycosylation. (E) Lectin western blot analysis revealing global glycosylation patterns in cell lysates from ALL patient 3 and control CLL as well as healthy donor blood cell lysates. B-ALL, B-cell ALL; PE, phycoerythrin.
CD81 loss in blasts from ALL patient 3 disrupts CD19 membrane trafficking. (A) Reverse transcription polymerase chain reaction (RT-PCR) analysis of CD19 mRNA expression in ALL patient 3 and primary CLL+ control cells as well as PC-3 and LNCap prostate negative control cell lines. (B) Schematic representation of CD81-dependent CD19 cell surface transport via endoplasmic reticulum (ER) and Golgi compartments. (C) Flow cytometric analysis of CD81 membrane expression on blasts from ALL patient 3 and healthy donor CD19+ B cells. (D) CD19 western blot analysis using lysates from ALL patient 3 and control CLL cell lysates ± PNGaseF treatment of deglycosylation. (E) Lectin western blot analysis revealing global glycosylation patterns in cell lysates from ALL patient 3 and control CLL as well as healthy donor blood cell lysates. B-ALL, B-cell ALL; PE, phycoerythrin.
As antigen-directed immunotherapies are translated into routine clinical practice, we need to shape our ideas about resistance mechanisms in order to optimize our treatments. Salvage strategies may range from broad immunotherapeutic approaches (such as immune checkpoint inhibition) in CD19+ relapses to CAR T cells or BiTEs with specificity for alternative leukemia-specific moieties in CD19− relapses or with specificity for alternative CD19 epitopes in CD19-truncated relapses. In view of the important percentage of patients developing epitope-loss escape variants, we should even consider venturing on dual antigen-targeting approaches in the first place to prevent resistance (eg, tandem CAR T cells or triple bodies). However, these concepts clearly need further studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christiane Horn for technical support.
M.B. holds a professorship for immunological cancer research supported by the Hubertus Wald Foundation.
Authorship
Contribution: M.B. designed the study, interpreted data, and wrote the manuscript; F.B., A.-K.K., and P.N. performed experiments; A.B. collected and analyzed clinical data and wrote the manuscript; M.G., H.-P.T., S.B., and R.C.B. collected and interpreted data; T.B. supplied vital reagents and interpreted data; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mascha Binder, Department of Oncology and Hematology, University Medical Center Hamburg-Eppendorf, Martinistr 52, D-20246 Hamburg, Germany; e-mail: m.binder@uke.de.
References
Author notes
F.B. and A.B. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal