To the editor:
Mutations in the tetratricopeptide repeat domain 7A (TTC7A) gene cause a severe form of very early onset inflammatory bowel disease (VEOIBD).1 TTC7A has a crucial role in chaperoning the enzyme phosphatidylinositol-4-kinase-3-α from the trans-Golgi apparatus to the plasma membrane to facilitate phosphorylation of phosphatidylinositol (PI). The composition of the plasma membrane in particular levels of phosphorylated PI (PI-4P) are crucial for preserving epithelial cell polarity and survival.1-3 The clinical spectrum of the disease varies from multiple intestinal atresias (MIA) to severe autoimmune enterocolitis clinically evident by infantile-onset intestinal obstruction/failure, bleeding, and diarrhea.1,4-6 Furthermore, the disease can be associated with severe immunodeficiency or autoimmune phenomena owing to the central role of TTC7A in thymic architecture.7 Limited published data on TTC7A-deficient patients suggest a median survival of <12 months of age.1,4-9 Allogeneic hematopoietic stem cell transplantation (HSCT) represents a possible treatment option for therapy-refractory VEOIBD with or without genetic diagnosis, especially when associated with immunological impairment.10-13 Moreover, evidence on the interaction between hematopoietic donor and host epithelial cells in stem cell transplantation might lead to a potential benefit of HSCT in certain epithelial disorders.14-16 TTC7A-deficient patients with associated immunodeficiency may therefore benefit from HSCT; however, the effect of engrafted donor cells on chronic intestinal inflammation and gut epithelial tissue regeneration is unknown.14,15 Follow-up data on HSCT in TTC7A deficiency are required to understand the impact of this treatment on the disease phenotype and to guide the management of future cases. In this study, we report the clinical and histological evolution of 4 TTC7A-deficient patients who are alive 19 to 114 months post-HSCT.
Anthropometric data, clinical features, and laboratory results from affected patients were extracted from clinical notes and prospective databases. One of the 4 patients included in this study was previously reported with shorter post-HSCT follow-up.6 We recruited candidates for molecular evaluation, including next-generation sequencing, as part of the PETIT Study (Patients with Early Onset Intestinal Inflammation Study). The molecular diagnosis was established through whole exome sequencing in 3 patients (in 1 case as previously reported6 ; for the other 2, Beckman Coulter Genomics on Illumina HiSeq2000, SureSelect Human All Exon Kit Version 4 Agilent Technologies, alignment: Burrows-Wheeler Aligner software, refinement: Genome Analysis Tool Kit) and targeted sequencing in 1 patient (polymerase chain reaction according to standardized diagnostic laboratory criteria; protocol provided upon request). Intestinal biopsies were fixed, paraffin embedded, and stained (hematoxylin and eosin) according to standardized diagnostic laboratory criteria (protocol provided upon request). Patients were informed and consented to functional studies and genetic sequencing as part of the PETIT Study. Ethical approval was obtained from the National Research Ethics Service Committee London, Bloomsbury. Between 2005 and 2013, 3 patients with TTC7A deficiency were treated at Great Ormond Street Hospital (London, United Kingdom), and 1 patient was treated at Boston Children’s Hospital (Boston, MA; extended medical histories are in the supplemental Material, available on the Blood Web site). In 1 case, a family history of early infantile deaths because of obstructive intestinal disease in the patient’s siblings was established. All children were diagnosed antenatally with bowel obstruction, required multiple surgeries in the neonatal period to address gut strictures, and were dependent on parenteral nutrition (PN) within the first 6 months of life. In 3 patients, attempts to control intestinal inflammation by deploying multiple immunosuppressant agents were undertaken without consistent improvement. There was evidence of immune dysfunction/dysregulation in all patients varying from severe T- and natural killer (NK)–cell deficiency with impaired mitogen response and low T-cell receptor excision circles (TRECs) to mild TREC impairment (Table 1; detailed in supplemental Tables 3 and 4). Patients received HSCT after reduced toxicity conditioning or serotherapy alone at 3, 9, 14, and 17 months of life (Table 1). At the time of writing, all children were alive at 19, 50, 66, and 114 months post-HSCT. Donor engraftment was complete in 1 and mixed in 3 patients with normal immune reconstitution in all children (Table 1; detailed in supplemental Table 3). Three patients required major surgical revisions for ongoing gut strictures post-HSCT, and 2 are still on immunosuppression. None of the patients could be weaned off PN, and 2 are currently listed for multivisceral transplantation because of additional liver dysfunction. Intestinal inflammation and abnormal epithelial features persisted beyond HSCT in all children (supplemental Material; supplemental Table 1) consistent with ongoing primary disease as opposed to a possible allogeneic phenomenon (ie, gut GVHD). Post-HSCT, 1 patient developed severe sensorineural deafness and immune-mediated hypothyroidism, 1 developed “flaky skin” phenotype and lung dysfunction of unclear origin. In 3 children, advanced liver disease with bridging fibrosis possibly because of long-term PN was established.
Characteristics of patients with TTC7A deficiency undergoing HSCT
| Genotype . | Immunology . | Gut phenotype . | IS pre-HSCT . | Donor . | Stem cell source . | Conditioning regimen . | GVHD . | Chimerism . |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | ||||||||
| p.Glu191fs | Low IgG levels | Pyloric stenosis | Steroids | Unrelated | CB | TBI 2 Gy | None | CD3 = 51% donor |
| p.I854Phe | Impaired mitogen stimulation | Microcolon | Azathioprine | HLA 8/10 | Cyclophosphamide 50 mg/kg | CD15 = 15% donor | ||
| Autoimmune enterocolitis (PE) | Infliximab | Fludarabine 200 mg/m2 | ||||||
| Daclizumab | ATG 7.5 mg/kg | |||||||
| Patient 2 | ||||||||
| p.Gly45_Ala55del | Low IgG levels | D/AC/TC atresias | Steroids | Sibling | BM | Treosulfan 42 g/m2 | Acute skin | CD3 = 100% donor |
| p.Gly45_Ala55del | Reduced TRECs | Microcolon | Infliximab | HLA 10/10 | Cyclophosphamide 120 mg/kg | Grade 1 | CD15 = 100% donor | |
| Autoimmune enterocolitis (PE) | Basiliximab | |||||||
| Cyclosporine | ||||||||
| Patient 3 | ||||||||
| p.Glu71Lys | Low IgG levels | Exomphalos | Steroids | Unrelated | PBSCs | Treosulfan 36 g/m2 | None | CD3 = 96% donor |
| p.Glu96 | Low T/NK cells | Pyloric/ileal atresias | HLA 12/12 | Fludarabine 150 mg/m2 | CD15 = 7% donor | |||
| Reduced TRECs | Microcolon | Alemtuzumab 1 mg/kg | ||||||
| Abnormal mitogen stimulation | Autoimmune enterocolitis (PE) | |||||||
| Patient 4 | ||||||||
| p.K606R | Low IgG levels | Pyloric stenosis | None | Sibling | BM | Equine ATG 30 mg/kg | None | CD3 = 100% donor |
| p.S672P | Low T cells | Jejunal and colonic atresias | HLA 9/10 | CD15 = 6% donor | ||||
| Absent TRECs | Microcolon | |||||||
| Abnormal mitogen stimulation |
| Genotype . | Immunology . | Gut phenotype . | IS pre-HSCT . | Donor . | Stem cell source . | Conditioning regimen . | GVHD . | Chimerism . |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | ||||||||
| p.Glu191fs | Low IgG levels | Pyloric stenosis | Steroids | Unrelated | CB | TBI 2 Gy | None | CD3 = 51% donor |
| p.I854Phe | Impaired mitogen stimulation | Microcolon | Azathioprine | HLA 8/10 | Cyclophosphamide 50 mg/kg | CD15 = 15% donor | ||
| Autoimmune enterocolitis (PE) | Infliximab | Fludarabine 200 mg/m2 | ||||||
| Daclizumab | ATG 7.5 mg/kg | |||||||
| Patient 2 | ||||||||
| p.Gly45_Ala55del | Low IgG levels | D/AC/TC atresias | Steroids | Sibling | BM | Treosulfan 42 g/m2 | Acute skin | CD3 = 100% donor |
| p.Gly45_Ala55del | Reduced TRECs | Microcolon | Infliximab | HLA 10/10 | Cyclophosphamide 120 mg/kg | Grade 1 | CD15 = 100% donor | |
| Autoimmune enterocolitis (PE) | Basiliximab | |||||||
| Cyclosporine | ||||||||
| Patient 3 | ||||||||
| p.Glu71Lys | Low IgG levels | Exomphalos | Steroids | Unrelated | PBSCs | Treosulfan 36 g/m2 | None | CD3 = 96% donor |
| p.Glu96 | Low T/NK cells | Pyloric/ileal atresias | HLA 12/12 | Fludarabine 150 mg/m2 | CD15 = 7% donor | |||
| Reduced TRECs | Microcolon | Alemtuzumab 1 mg/kg | ||||||
| Abnormal mitogen stimulation | Autoimmune enterocolitis (PE) | |||||||
| Patient 4 | ||||||||
| p.K606R | Low IgG levels | Pyloric stenosis | None | Sibling | BM | Equine ATG 30 mg/kg | None | CD3 = 100% donor |
| p.S672P | Low T cells | Jejunal and colonic atresias | HLA 9/10 | CD15 = 6% donor | ||||
| Absent TRECs | Microcolon | |||||||
| Abnormal mitogen stimulation |
Additional data on histological evolution post-HSCT are in the supplemental Material.
ATG, antithymocyte globulin; BM, bone marrow; CB, cord blood; D/AC/TC, duodenum/ascending colon/transverse colon; GVHD, graft-versus-host disease; IgG, immunoglobulin G; IS, immune suppression; PBSC, peripheral blood stem cell; PE, panenteric (small and large bowel); TBI, total body irradiation.
Published data on TTC7A deficiency are scarce. Overall, 52 cases with genetically confirmed TTC7A deficiency were reported in the literature.3,6-9,15,16 Clinical outcomes are available for 45 patients, 30 of whom have died (median age at death 8 months). Fifteen reported patients are alive at a median age of 27 months. All of them have a severe gastrointestinal disease (severe diarrhea, gut failure, and MIA), 6 are PN dependent, and 11 show signs of immunodeficiency and/or autoimmune disorders.1,6-8 Most of the reported patients were treated symptomatically and managed with supportive medical and surgical care. One reported case was referred for combined small intestine and HSCT but was lost to follow-up.6 Six published patients underwent HSCT, of whom 5 died within the first year postprocedure (3 from infections, 1 from disease progression, and 1 from unknown causes).6,8,9,15 The only survivor is reported here as patient 4.
More than 20 private mutations in the TTC7A gene have been described ranging from homozygous deletions as demonstrated in the first publication associating the TTC7A gene with MIA4 to compound heterozygous missense mutations.1,6,8,9 There is insufficient evidence to suggest a correlation between type or position of the mutation within the TTC7A gene and clinical severity. This is confirmed by our findings. Survival and clinical and histological evolution of the disease appeared to be similar in our patients despite markedly different genotypes. Patient 2 harbored a large homozygous deletion in exon 1 (Table 1; Figure 1) leading to a premature stop codon at the start of exon 2 (amino acid position 67) rendering the presence of a functional TTC7A protein highly unlikely. Considering that 3 patients in our cohort underwent HSCT at a higher median age than the reported median age of death in the literature, we suggest that disease severity plays a central role in early patient survival and eligibility for HSCT. All our patients are alive post-HSCT, suggesting that reduced or minimal toxicity conditioning is a critical point to avoid transplantation-related mortality and still obtain T-cell engraftment. Our findings suggest that although HSCT was feasible and safe, correcting the immune dysfunction through HSCT in TTC7A patients did not influence the epithelial phenotype or promote enteral tolerance. Restoring immunocompetence in patients with concomitant immunodeficiency may nevertheless increase the chance for long-term survival limiting infection-related comorbidities across multiple surgeries and long-term PN. The role of HSCT in preventing immune-mediated phenomena, which appear later in life for TTC7A-deficient patients has yet to be established and is therefore difficult to include in the decision-making process.7 Small bowel transplantation in genetically confirmed TTC7A deficiency has not yet been reported, but given the overall improving results of intestinal transplantation in children, this might be an option in selected cases.17,18
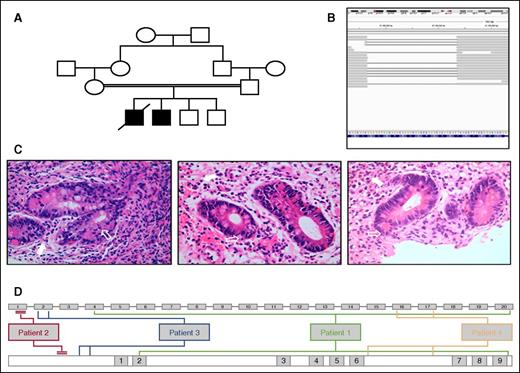
Patients' characteristics. (A) Pedigree for patient 2 with TTC7A deficiency. (B) Integrative Genomics Viewer of whole exome sequencing data from patient 2. Broad gray bars represent sequenced reads aligned to the reference genome. Thin gray bars represent missing/deleted segments (large 34-bp deletion demonstrated in exon 1, confirmed on Sanger sequencing). (C) Hematoxylin and eosin–stained gut biopsies taken pre-HSCT and 6 months and 18 months post-HSCT (from left to right) from patient 2. Unfilled white arrows indicate apoptotic debris. Filled white arrows indicate infiltration of the lamina propria with lymphocytes, eosinophils, plasma cells, and neutrophils. (D) Position of all causative mutations within TTC7A gene (top line: exon 1-20) and position within TTC7A protein (lower line: tetratricopeptide repeat domains 1-9) for all 4 patients.
Patients' characteristics. (A) Pedigree for patient 2 with TTC7A deficiency. (B) Integrative Genomics Viewer of whole exome sequencing data from patient 2. Broad gray bars represent sequenced reads aligned to the reference genome. Thin gray bars represent missing/deleted segments (large 34-bp deletion demonstrated in exon 1, confirmed on Sanger sequencing). (C) Hematoxylin and eosin–stained gut biopsies taken pre-HSCT and 6 months and 18 months post-HSCT (from left to right) from patient 2. Unfilled white arrows indicate apoptotic debris. Filled white arrows indicate infiltration of the lamina propria with lymphocytes, eosinophils, plasma cells, and neutrophils. (D) Position of all causative mutations within TTC7A gene (top line: exon 1-20) and position within TTC7A protein (lower line: tetratricopeptide repeat domains 1-9) for all 4 patients.
The online version of the article contains a data supplement.
Authorship
Contribution: J.K., D.E., and H.U. were in charge of the genetic diagnosis and analysis for the reported patients; J.K., G.L., and P.V. wrote the manuscript; S.-Y.P., A.W., P.A., J.S., R.C., K.R., G.N.-J., M.G., M.E., N. Shah, L.N., and P.V. were in charge of the clinical management of the patients pre-, during, and posttransplant and collected and made the follow-up data available for the study; and D.R. and N. Sebire analyzed the longitudinal histopathology data from the patients.
Conflict-of-interest disclosure: H.U. is supported by the Crohn’s & Colitis Foundation of America and the Leona M. and Harry B. Helmsley Charitable Trust. The remaining authors declare no competing financial interests.
Correspondence: Paul Veys, Department of Immunology and Bone Marrow Transplantation, Great Ormond Street Hospital, London WC1N 3JH, United Kingdom; e-mail: paul.veys@gosh.nhs.uk.
References
Author notes
J.K. and G.L. contributed equally to this study.
N. Shah and P.V. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal