Key Points
Acute GVHD leads to defective MHC class II antigen presentation by donor DC, leading to a failure of peripheral Treg homeostasis.
Impaired Treg homeostasis results in chronic GVHD directly and can be alleviated by adoptive Treg transfer.
Abstract
Chronic graft-versus-host disease (cGVHD) is a major cause of late mortality following allogeneic bone marrow transplantation (BMT) and is characterized by tissue fibrosis manifesting as scleroderma and bronchiolitis obliterans. The development of acute GVHD (aGVHD) is a powerful clinical predictor of subsequent cGVHD, suggesting that aGVHD may invoke the immunologic pathways responsible for cGVHD. In preclinical models in which sclerodermatous cGVHD develops after a preceding period of mild aGVHD, we show that antigen presentation within major histocompatibility complex (MHC) class II of donor dendritic cells (DCs) is markedly impaired early after BMT. This is associated with a failure of regulatory T-cell (Treg) homeostasis and cGVHD. Donor DC-restricted deletion of MHC class II phenocopied this Treg deficiency and cGVHD. Moreover, specific depletion of donor Tregs after BMT also induced cGVHD, whereas adoptive transfer of Tregs ameliorated it. These data demonstrate that the defect in Treg homeostasis seen in cGVHD is a causative lesion and is downstream of defective antigen presentation within MHC class II that is induced by aGVHD.
Introduction
Allogeneic bone marrow transplantation (BMT) is an effective curative therapy for the majority of hematologic malignancies. The curative property of BMT lies mainly the immune-mediated graft-versus-leukemia effect, which eradicates residual recipient leukemia by T and natural killer (NK) cells.1,2 The wider application of this treatment is limited by serious posttransplantation complications, including graft-versus-host disease (GVHD). GVHD occurs in both acute (aGVHD) and chronic forms (cGVHD) and develops when the donor graft recognizes the recipient as foreign and elicits an immune response. Granulocyte-colony stimulating factor (G-CSF) mobilized peripheral blood (G-CSF PB) is now the predominant stem cell source for allogeneic BMT despite the clear association with a significant increase in incidence and severity of cGVHD.3 Unfortunately there are few effective therapies available for cGVHD; thus, an enhanced understanding of the pathophysiology of cGVHD is required to allow the rational development of new therapeutics.
The induction of acute and chronic forms of GVHD differs temporally and mechanistically. cGVHD manifests as a diverse range of multiorgan pathologies; however, the skin, mouth, liver, and eyes are most commonly affected.4 cGVHD presents with features common to autoimmune diseases such as systemic lupus erythematosus and systemic sclerosis in which pathology is characterized by fibrosis.5 In contrast, the defining feature of aGVHD is apoptosis. The most powerful predictor of cGVHD is preceding aGVHD, although it can also manifest in patients who have not displayed prior aGVHD.6 Although aGVHD is critically dependent on alloreactive donor T cells contained within the graft, their contribution to cGVHD is less clear, and there is evidence for a role for autoreactive T cells in the process.7-11
Regulatory T cells (Tregs) play an important role in the control of innate and adaptive immune responses. The absence or dysfunction of Tregs is known to lead to the development of autoimmune diseases,12 and it is proposed that a perturbation of this population contributes to the pathology observed in aGVHD and cGVHD. In this regard, the number of Tregs in the donor graft has been inversely correlated with aGVHD both clinically and in preclinical animal models.13-16 Furthermore, in both preclinical and clinical studies, cGVHD is associated with decreased numbers of circulating Tregs.17-20 However, the factors contributing to diminished Treg numbers in the setting of cGVHD and the mechanism by which their absence contributes to pathology remain to be elucidated, although enhanced Treg proliferation and Fas-dependent cell death clearly contribute.20
A role for conventional dendritic cells (cDCs) in the induction and maintenance of Tregs in general is well established,21 and the importance of donor DC interactions with Treg after transplant has recently been reported.22,23 Previous studies from our laboratory have identified cDC as the critical donor antigen-presenting cell (APC) for host antigen presentation after transplant and that this contributes to the perpetuation of aGVHD.24-26 Recently, we demonstrated a specific impairment in major histocompatibility complex (MHC) class II antigen presentation by cDC is induced by the inflammatory cytokine milieu generated during aGVHD.27 In contrast to our increasing appreciation of the contribution of donor cDC to aGVHD, their role in cGVHD remains relatively unexplored. We hypothesized that the failure of donor MHC class II antigen presentation by cDCs may lead to a failure in Treg development late after transplant, resulting in a loss of tolerance and cGVHD.
Methods
Stem cell transplantation
On day 0, recipient mice received 1100 (B6D2F1), 1000 (B6), or 900 cGy (FVB/N) total body irradiation (TBI) split into 2 doses separated by 3 hours. On day −2 prior to TBI, FVB/N mice were treated with NK cell depletion (1 mg NK1.1 intraperitoneally). T-cell depletion (TCD) of donor bone marrow (BM) cells and purification of splenic CD3+ T cells were performed as previously described,28 generating TCD BM with <1% contaminating CD3+ T cells and CD3+ T-cell preparations of >85% purity. Recipients were transplanted with 5 × 106 (B6D2F1, FVB/N) or 10 × 106 (B6) TCD BM cells from B6, B6D2F1, BALB/c, B6.CD11c.OVA, B6.H2-Ab1−/−, B6.CD11c Creneg, or I-Abflox/flox xCD11c-Cre (I-AbCD11c-Crepos) and B6 or BALB/c.FoxP3-DTR-GFP donor mice as indicated, with or without supplementation with CD3+ T cells from the same donors or, where indicated, from B6.FoxP3-luc+ or B6.luc+ donors. Transplanted mice were monitored daily, and those with GVHD clinical scores29 ≥6 were killed, and the date of death registered as the next day in accordance with institutional animal ethics committee guidelines.
Treg depletion and transfers in vivo
From day 14 after BMT, recipients were injected intraperitoneally with 100 ng diphtheria toxin (DT; Sigma-Aldrich) twice weekly (Tuesday and Friday) for 6 weeks. BALB/c (CD45.1+) splenic CD4+ T cells were purified using magnetic activated cells sorting (Miltenyi Biotec). Tregs were then sorted on the basis of CD4+, CD25+, and 7AAD− expression using the FACSAria (BD Bioscience). The postsort purity was >95% FoxP3+ and each recipient received 0.5 × 106 sorted cells intravenously.
In vivo bioluminescence imaging
Anesthetized mice were injected with firefly luciferin (500 µg; Caliper Life Sciences) subcutaneously and imaged 5 minutes later with the Xenogen imaging system (Xenogen IVIS 100; Caliper Life Sciences). Data were analyzed with Living Image Version 4 software (Xenogen) and presented as photons per second per square centimeter per steer radiant (ph s−1 cm−2 sr−1).
Statistical analysis
Data are shown as mean ± standard error of the mean. A 2-tailed Mann-Whitney U test was used for group comparisons unless otherwise indicated. Survival plots were estimated and plotted by Kaplan-Meier methods, and difference between subgroups was estimated by log-rank methods (*P < .05; **P < .01; ***P < .001; ****P < .0001; GraphPad Prism 6.01).
See supplemental Data, available on the Blood Web site, for additional methods.
Results
Chronic GVHD is associated with reduced Treg numbers and immune suppression
The pathophysiology of cGVHD is complex and shares features with autoimmune diseases. cGVHD can be preceded by aGVHD; however, it does also manifest in patients who have not displayed symptoms of aGVHD (so called de novo cGVHD). We first established models of cGVHD in which sclerodermatous pathology developed in the skin late after transplant following a period of low-level aGVHD. In the first model, using BALB/c donors and C57BL/6 recipients (Figure 1A), a lower dose of donor T cells (2 × 106) was introduced in the graft and compared with a higher T-cell dose (5 × 106) established to induce aGVHD morbidity and mortality. Recipients of lower-dose T cells demonstrated mild to moderate aGVHD with clinical scores between 2 and 4 from day 14 following BMT (Figure 1B) with no associated mortality (data not shown). In contrast, all recipients of the higher T-cell dose succumbed to GVHD by day 30, whereas recipients of grafts devoid of T cells (non-GVHD controls) did not develop GVHD and survived long term. As clinical cGVHD is associated with a defect in Tregs, we quantified the frequency and absolute number of splenic CD4+FoxP3+ Tregs in transplant recipients on day 28 after BMT. As predicted, the frequency and number (although the latter did not reach significance) of CD4+FoxP3+ Tregs was reduced in proportion to the donor T-cell dose in the BM graft and thus the severity of GVHD (Figure 1C). Similarly, in the B6→B6D2F1 model (Figure 1D), the severity of GVHD, as determined by clinical scores, was dependent on the donor T-cell dose (Figure 1E), with only mild to moderate GVHD induced with no associated mortality (data not shown), in response to the low T-cell doses administered. Notably, compared with non-GVHD controls, there was a profound decrease in CD4+FoxP3+ Treg frequency and absolute numbers at all donor T-cell doses examined (Figure 1F), suggesting that Treg homeostasis is exquisitely sensitive to even subtle levels of aGVHD. Finally, the development of sclerodermatous GVHD was confirmed in both models (Figure 1G-H; supplemental Figure 2A). Additionally, further histopathologic characterization of GVHD target organs in the B6 → B6D2F1 model demonstrated collagen deposition (fibrosis) in the lung, whereas inflammation and architectural disorganization characterized pathology in the liver and salivary gland (supplemental Figure 2B). Thus, cGVHD pathology in this model recapitulates the spectrum of cGVHD pathology that manifests clinically.
Chronic GVHD is associated with reduced Treg cell numbers and immune suppression. (A) Lethally irradiated C57BL/6 mice were transplanted with 10 × 106 TCD BM cells and no T cells or 2 × 106 or 5 × 106 CD3+ T cells from BALB/c donors (n = 4-12, 2 experiments). (B) Clinical scores of the recipients. (C) Frequency and number of splenic CD3+CD4+FoxP3+ were analyzed at day 28 (n = 1-6, 1 experiment). (D) Lethally irradiated B6D2F1 mice received BMT with 5 × 106 TCD BM cells and no T cells or 0.1, 0.25, or 0.5 × 106 CD3+ T cells from C57BL/6 donors (n = 8, 2 experiments). (E) Clinical scores of the recipients. (F) Frequency and number of splenic CD3+CD4+FoxP3+ were analyzed at day 28 (n = 3, 1 experiment). Representative images of H&E staining of skin taken at day 28 after BMT of the recipients from the BMT described in (G) A (T-cell dose: 3 × 106) and (H) D (T-cell dose: 0.5 × 106). (I) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells from WT.B6 or B6.CD11c.OVA donor mice with or without 0.5 × 106 CD3+ T cells from C57BL/6 donor mice. At day 28 after BMT, CFSE-labeled and -sorted OT-II Tg T cells (2 × 106 cells) were injected in intravenous CFSE dilution and analyzed 3 days later in spleen. The index of proliferation is represented (n = 5-11) from 2 experiments. A 1-way analysis of variance was performed to compare mean proliferative indexes between groups. (J) Lethally irradiated B6D2F1 or FVB/N recipients were transplanted with 5 × 106 TCD BM cells from B6 or B6D2F1, respectively, with or without supplementation with CD3+ T cells from the same donor. Splenic donor cDC reactivity with YAe antibody was assessed by flow cytometry on day 28 after BMT. t test with Welch’s correction was performed for each system. *P < .05; **P < .01; ***P < .001; ****P < .0001 (GraphPad Prism 6.01).
Chronic GVHD is associated with reduced Treg cell numbers and immune suppression. (A) Lethally irradiated C57BL/6 mice were transplanted with 10 × 106 TCD BM cells and no T cells or 2 × 106 or 5 × 106 CD3+ T cells from BALB/c donors (n = 4-12, 2 experiments). (B) Clinical scores of the recipients. (C) Frequency and number of splenic CD3+CD4+FoxP3+ were analyzed at day 28 (n = 1-6, 1 experiment). (D) Lethally irradiated B6D2F1 mice received BMT with 5 × 106 TCD BM cells and no T cells or 0.1, 0.25, or 0.5 × 106 CD3+ T cells from C57BL/6 donors (n = 8, 2 experiments). (E) Clinical scores of the recipients. (F) Frequency and number of splenic CD3+CD4+FoxP3+ were analyzed at day 28 (n = 3, 1 experiment). Representative images of H&E staining of skin taken at day 28 after BMT of the recipients from the BMT described in (G) A (T-cell dose: 3 × 106) and (H) D (T-cell dose: 0.5 × 106). (I) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells from WT.B6 or B6.CD11c.OVA donor mice with or without 0.5 × 106 CD3+ T cells from C57BL/6 donor mice. At day 28 after BMT, CFSE-labeled and -sorted OT-II Tg T cells (2 × 106 cells) were injected in intravenous CFSE dilution and analyzed 3 days later in spleen. The index of proliferation is represented (n = 5-11) from 2 experiments. A 1-way analysis of variance was performed to compare mean proliferative indexes between groups. (J) Lethally irradiated B6D2F1 or FVB/N recipients were transplanted with 5 × 106 TCD BM cells from B6 or B6D2F1, respectively, with or without supplementation with CD3+ T cells from the same donor. Splenic donor cDC reactivity with YAe antibody was assessed by flow cytometry on day 28 after BMT. t test with Welch’s correction was performed for each system. *P < .05; **P < .01; ***P < .001; ****P < .0001 (GraphPad Prism 6.01).
We previously demonstrated that aGVHD, early after BMT, corrupts the capacity of donor conventional DC (cDC: CD11chiMHC class II+) to present antigen within MHC class II.27 We therefore investigated whether a defect in antigen presentation occurred in these systems later after BMT, when cGVHD was developing. We used the B6→B6D2F1 model whereby the donor graft consisted of BM derived from CD11c.OVA transgenic (Tg) donors (ovalbumin [OVA] driven off the CD11c promoter; predominantly expressed by cDCs) or wild-type B6 (B6.WT) donors together with low doses of wild-type B6 T cells (0.5 × 106). At day 28 after BMT, carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled OT-II TCR Tg reporter T cells (which specifically recognize the OVA(323-339)-derived peptide presented in MHC class II) were adoptively transferred into recipients. Three days later, CFSE dilution in adoptively transferred OT-II Tg T-cells was assessed to quantify antigen presentation by donor cDC. OT-II Tg T cells failed to proliferate in recipients of B6.WT grafts (with or without T cells), confirming the specificity of the OT-II response to donor MHC/ova peptide complex and negating a contribution from homeostatic proliferation in TCD recipients. In contrast, OT-II Tg T cells proliferated robustly in recipients of CD11c.OVA TCD BM grafts, but this was markedly attenuated by the addition of T cells to the graft and consequent GVHD (Figure 1I). Of note, at the time of transfer, the OT-II cells are healthy and are only exposed to a GVHD environment for a limited period of time; thus, these assays primarily reflect Ag presentation. Indeed we previously demonstrated that the proliferation of third party (DBA/1) T cells in these systems was not impaired and thus the effect is not due to proliferation defects.27 As we have recently reported, GVHD increases MHC class II expression on donor cDCs,27,30 which we confirmed here at this late time point (data not shown). Thus, altered MHC expression does not explain the impaired cDC function in the setting of GVHD. We therefore next sought to ascertain the predominant source of antigen (ie, donor vs host) being presented by donor DC at D28 after transplant and enumerate the number of donor DC presenting these antigens. We used the YAe antibody that detects an MHC class II–derived peptide (Ea peptide) loaded on MHC class II (I-Ab) and 2 models of GVHD (B6→B6D2F1 and B6D2F1→FVB/N) in which the Ea peptide could only be derived from the host (allo-antigen) or donor (self-antigen) compartments, respectively. Thus, the systems are set up such that the peptide can originate from recipient and be presented by donor (B6→B6D2F1) or originate from and be presented by donor (B6D2F1→FVB). Notably, we confirmed the development of sclerodermatous skin GVHD with associated liver, lung, and salivary gland pathology (supplemental Figure 3) in the B6D2F1→FVB/N model. As previously noted, the cDC expression of MHC class II was upregulated (data not shown). Furthermore, we demonstrate a significantly increased number of YAe+ donor DC expressing self-antigen compared with allo-antigen expressing donor DCs (>100 fold) and that GVHD significantly reduced the number of both (Figure 1J). Importantly, not only was the absolute number of DC presenting donor Ag reduced, but also their antigen presenting capacity as reflected by a reduced proportion of YAe-positive DCs in the donor DC compartment (supplemental Figure 5A). In the system measuring recipient Ag presentation by donor DCs, there is a decrease in absolute number of splenic donor DCs during GVHD (supplemental Figure 5B), but this was not significant in the GVHD system determining presentation of donor Ags. This likely reflects relative differences in levels of GVHD in the 2 systems. Thus, the reduction in presentation of donor Ags during GVHD is predominantly due to reduced levels of Ag rather than numbers of donor DCs. Interestingly, the presentation of alloantigen in the gastrointestinal tract was increased in animals with GVHD (supplemental Figure 5D), consistent with recent observations demonstrating the ability of donor colonic DC to expand effector T cells at this site.24 It is also important to note that in the spleen the proportion of donor DC presenting host alloantigen is very small and this is in contrast to the same alloantigen presentation by donor DCs in the mesenteric lymph nodes (supplemental Figure 5C) as previously reported.24 The total number of donor DCs in the mesenteric lymph nodes, however, is not affected by the subclinical levels of GVHD late after BMT in these models (supplemental Figure 5D). Thus, in these systems, the primary impairment of Ag presentation in the periphery during GVHD appears to be of donor rather than recipient antigen. We therefore demonstrate significant impairment of allo- and especially self-antigen presentation, demonstrated here with the YAe peptide, which recapitulated the observed defective antigen presentation of the Ova derived peptide (323-339; as determined by OTII proliferation; Figure 1I) by donor DC in the periphery during GVHD.
MHC II deficiency within donor cDCs induces Treg deficiency and cGVHD
Having demonstrated a diminished MHC class II antigen presentation and a significant reduction in CD4+FoxP3+ Tregs during cGVHD, we next sought to causally link these 2 observations. Previous studies demonstrated that BMT recipients of MHC class II–deficient BM developed cGVHD pathology, which was prevented by thymectomy.9 These studies demonstrated that T cells escaping thymic negative selection were self-reactive and able to mediate cGVHD in secondary recipients. Although this establishes that BM derived T cells were responsible for cGVHD, it does not ascertain whether the pathologic T-cell response occurs in the thymus or at a later stage in the periphery (because T cells will be affected both centrally and peripherally). We hypothesize that the failure of donor MHC class II antigen presentation by cDCs in the presence of aGVHD leads to reduced Treg development late after transplant, leading to a loss of tolerance and the development of cGVHD. We thus investigated whether the absence of MHC class II within the BM graft leads to Treg deficiency and cGVHD. We determined the severity of cGVHD in B6D2F1 recipients transplanted with B6.WT or MHC class II–deficient (H2-Ab1−/−) TCD BM grafts. The recipients of H2-Ab1-/- TCD BM grafts indeed developed GVHD as reflected by increasing clinical scores from day 50 after transplant (Figure 2A). Examination of skin at day 70 after transplant confirmed the development of cGVHD9,31 and that MHC class II deficiency in the BM graft induced clear sclerodermatous-like pathology in the skin of these recipients (Figure 2B). Examination of splenic CD4+FoxP3+ Tregs at day 70 revealed a significant reduction in CD4+FoxP3+ Treg frequency and absolute number in H2-Ab1−/− TCD BM recipients compared with recipients of WT TCD BM (Figure 2C). These data support the notion that impaired MHC class II antigen presentation induces a failure of Treg homeostasis, potentially leading to the development of cGVHD.
MHC class II deficiency induces chronic GVHD with associated reduced Treg number. (A) Clinical scores of lethally irradiated B6D2F1 recipients transplanted with 5 × 106 TCD BM cells from B6.H2-Ab1−/− or WT donor mice (n = 14-15, 3 experiments). (B) Representative images of H&E staining of skin taken at day 70 after BMT and GVHD histopathology scores quantified in the skin (n = 4-5, 1 experiment). (C) The frequency and number of CD3+CD4+FoxP3+ cells were analyzed in the spleen of the recipients at day 70 after BMT (n = 8-10, 2 experiments).
MHC class II deficiency induces chronic GVHD with associated reduced Treg number. (A) Clinical scores of lethally irradiated B6D2F1 recipients transplanted with 5 × 106 TCD BM cells from B6.H2-Ab1−/− or WT donor mice (n = 14-15, 3 experiments). (B) Representative images of H&E staining of skin taken at day 70 after BMT and GVHD histopathology scores quantified in the skin (n = 4-5, 1 experiment). (C) The frequency and number of CD3+CD4+FoxP3+ cells were analyzed in the spleen of the recipients at day 70 after BMT (n = 8-10, 2 experiments).
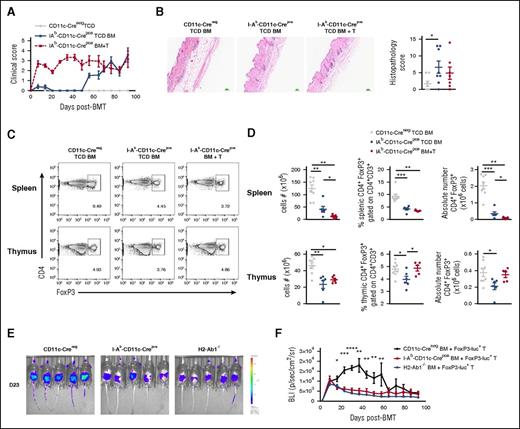
We next examined whether these effects were specifically due to defective MHC class II antigen presentation by donor cDCs by using I-Ab-CD11c-Crepos donors to induce cDC-specific MHC class II deficiency. The transplantation of BM from these recipients recapitulated the clinical and histologic phenotypes seen in the setting of global donor MHC class II deficiency (Figure 3A-B). Moreover, at day 93 after transplant, we noted a significant reduction in the frequency and absolute number of splenic CD4+FoxP3+ Tregs in BMT recipients lacking MHC class II within donor cDCs compared with the WT non-GVHD control group (CD11c-Creneg TCD BM) regardless of the presence of preexisting GVHD (Figure 3C-D). Importantly, the absence of MHC class II in donor cDCs combined with aGVHD resulted in a synergistic reduction in splenic donor Treg numbers. Although more marked in the spleen, donor DC MHC class II deficiency also resulted in a modest reduction in thymic Tregs in TCD recipients. However, when T cells were included in the grafts, the proportion and absolute number of thymic Tregs was similar to that of non-GVHD controls (Figure 3C-D). Thus, although the absence of MHC class II on donor cDCs within the thymus results in the generation of autoreactive donor T cells, ultimately responsible for cGVHD, the ability to control these aberrant T cells lies within the Treg pool, whose maintenance is dependent on MHC class II presentation by donor cDCs, predominantly in the periphery. To understand the mechanism of Treg failure in the absence of MHC class II antigen presentation specifically in cDCs, we tracked FoxP3+ Treg development in recipients transplanted with CD3+ T cells from B6.FoxP3-luc+ donor mice in which expression of the luciferase (luc) is driven off the FoxP3 promoter. We imaged the mice weekly and observed in the WT (CD11c-Creneg) recipients (with intact MHC class II antigen presentation), an increase in FoxP3-luc+ cells at day 14, which peaked around day 40, and then gradually decreased and disappeared by day 65 (Figure 3E-F). As expected, there was a failure in homeostasis within FoxP3-luc+ cells in the absence of MHC class II in cDCs. This homeostatic failure in Tregs was similar when all donor APCs lacked MHC class II, demonstrating the critical role for donor cDCs in maintaining Tregs.
MHC class II deficiency within cDCs induces Treg deficiency and cGVHD. (A) Clinical scores of lethally irradiated B6D2F1 recipients transplanted with 5 × 106 TCD BM cells with (BM+T) or without (TCD) 0.5 × 106 CD3+ T cells from CD11c Creneg or I-Ab CD11c Crepos donor mice (n = 8, 1 experiment). (B) Representative images of H&E staining of skin taken at day 93 after BMT and GVHD histopathology scores quantified in the skin (n = 6-8, 1 experiment). (C) Representative dot plots of CD4+FoxP3+ cells gated on CD3+CD4+ T cells in spleen and thymus at day 94 after BMT. (D) Cell numbers, frequency, and absolute numbers of CD3+CD4+FoxP3+ Treg cells in spleen and thymus (n = 5-8, 1 experiment). (E) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells from CD11c Creneg, I-Ab CD11c Crepos, or B6.H2-Ab1−/− donor mice with 0.5 × 106 FoxP3-luc+ CD3+ T cells. Representative images of the FoxP3-luc+CD3+ T cells are represented in the whole mice at day 23 after BMT. (F) The bioluminescence (BLI, ph s−1 cm−2 sr−1) is represented and followed over time after BMT (n = 10, 2 experiments). Each dot corresponds to 1 individual mouse.
MHC class II deficiency within cDCs induces Treg deficiency and cGVHD. (A) Clinical scores of lethally irradiated B6D2F1 recipients transplanted with 5 × 106 TCD BM cells with (BM+T) or without (TCD) 0.5 × 106 CD3+ T cells from CD11c Creneg or I-Ab CD11c Crepos donor mice (n = 8, 1 experiment). (B) Representative images of H&E staining of skin taken at day 93 after BMT and GVHD histopathology scores quantified in the skin (n = 6-8, 1 experiment). (C) Representative dot plots of CD4+FoxP3+ cells gated on CD3+CD4+ T cells in spleen and thymus at day 94 after BMT. (D) Cell numbers, frequency, and absolute numbers of CD3+CD4+FoxP3+ Treg cells in spleen and thymus (n = 5-8, 1 experiment). (E) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells from CD11c Creneg, I-Ab CD11c Crepos, or B6.H2-Ab1−/− donor mice with 0.5 × 106 FoxP3-luc+ CD3+ T cells. Representative images of the FoxP3-luc+CD3+ T cells are represented in the whole mice at day 23 after BMT. (F) The bioluminescence (BLI, ph s−1 cm−2 sr−1) is represented and followed over time after BMT (n = 10, 2 experiments). Each dot corresponds to 1 individual mouse.
Because the previous experiments demonstrated a critical role for donor cDCs in maintaining Tregs and preventing cGVHD, we next sought to understand the consequence of defective antigen presentation within MHC class II on effector T-cell (Teff) expansion and GVHD in the absence of thymic-derived Tregs (tTreg). We tracked Teff expansion by using CD3+ T-cells from B6.luc+ donors that were depleted of tTregs. We observed that the Teff in recipients of BM from both CD11c-Creneg and pos donors expanded maximally in the third week and also late after BMT (10 weeks) (Figure 4A-B). As previously demonstrated, maximal donor CD4 effector T-cell expansion early after BMT required MHC class II–restricted antigen presentation by donor DCs,24 but expansion thereafter could be maintained independently of donor DC MHC class II expression in animals with mild aGVHD. Taken together, our data demonstrate that antigen presentation within MHC class II by donor cDCs is critical to maintain and expand both mature donor CD4+FoxP3+ Tregs (contained within the graft) and BM-derived Tregs, which together prevent the development of cGVHD. Importantly, the homeostatic role of MHC class II antigen presentation in donor cDCs appears to occur predominantly in the periphery.
MHC class II antigen presentation by donor cDCs is not critical for Teff function in cGVHD. (A) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells from CD11c Creneg or I-Ab CD11c Crepos donor mice with 0.5 × 106 CD3+ T cells from B6.luc+ mice previously depleted in Tregs with the administration of the antibody PC61. (B) Representative images of the B6.luc+ CD3+ T cells are represented in the whole mice at day 23 after BMT. The BLI (ph s−1 cm−2 sr−1) is represented and followed over time every week after BMT (n = 14-15, 3 experiments).
MHC class II antigen presentation by donor cDCs is not critical for Teff function in cGVHD. (A) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells from CD11c Creneg or I-Ab CD11c Crepos donor mice with 0.5 × 106 CD3+ T cells from B6.luc+ mice previously depleted in Tregs with the administration of the antibody PC61. (B) Representative images of the B6.luc+ CD3+ T cells are represented in the whole mice at day 23 after BMT. The BLI (ph s−1 cm−2 sr−1) is represented and followed over time every week after BMT (n = 14-15, 3 experiments).
Donor Tregs prevent the generation of scleroderma
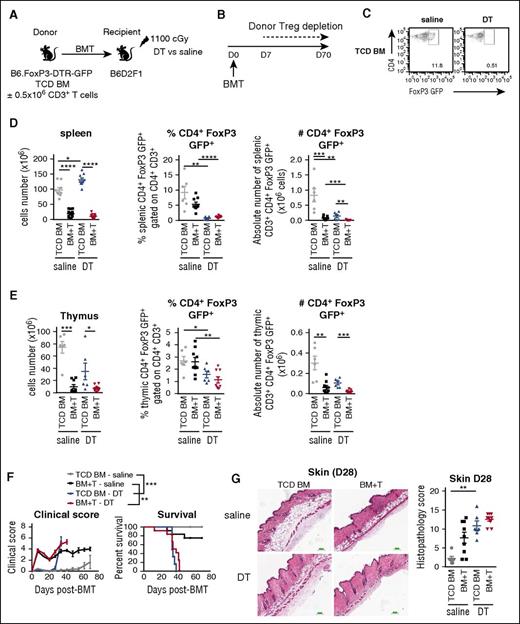
We next investigated whether there is a direct cause and effect relationship between increased CD4+FoxP3+ Treg numbers and protection from cGVHD. We used the B6→B6D2F1 model using low T-cell doses (0.5 × 106) to induce mild GVHD, and BM grafts from B6.FoxP3-DTR-GFP donor mice in which expression of the diphtheria toxin receptor (DTR) is driven off the FoxP3 promoter such that specific FoxP3+Treg depletion could be maintained by continued administration of DT (Figure 5A). Because we observed that donor CD4+FoxP3+ Tregs expand in the first week after BMT, we initiated depletion from day 7 and confirmed Treg depletion at day 28 (Figure 5B-C). As expected in the no DT groups, the cellularity of both spleen and thymus were significantly reduced in the presence of low-grade aGVHD and at this early time point (day 28) when the effects of aGVHD are more prominent, the number of CD4+FoxP3+ Treg was reduced in both of these organs (Figure 5D-E). Moreover, in recipients administered DT, Tregs were significantly reduced in both the spleen and thymus (Figure 5C-E), which was associated with late cGVHD lethality, regardless of the presence of donor T cells in the initial graft (median survival BM + T graft + DT: 37 days vs BM + T graft + saline: 70 days) (Figure 5F). Accordingly, all recipients in which Tregs were depleted developed scleroderma (Figure 5G). These data were confirmed in a second model using BALB/c-FoxP3-DTR-GFP donors transplanted into B6 recipients (supplemental Figure 4).
Donor Tregs prevent the generation of scleroderma. (A) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells with (BM+T) or without (TCD BM) 0.5 × 106 CD3+ T cells from B6.FoxP3-DTR-GFP donor mice. (B) After transplantation, the mice were injected intraperitoneally twice a week with saline or DT from day 7 for a period of 6 weeks. (C) Representative dot plots of CD4+FoxP3 GFP+ cells gated on CD3+CD4+ T cells in spleen after saline or DT treatment at day 28. The splenic cell number and the frequency and number of CD4+FoxP3 GFP+ cells within CD3+CD4+ T cells were analyzed in (D) spleen and (E) thymus at day 28 after BMT (n = 6-9). (F) Clinical scores and survival of the recipients from the BMT as described in A (**P < .01: BM+T-saline vs BM+T-cells-DT, ***P < .001: TCD BM-saline vs TCD BM-DT, 2 experiments). (G) Representative images of H&E staining of skin samples were taken at day 28 after BMT and quantified for GVHD histopathology (n = 6-9, 2 experiments).
Donor Tregs prevent the generation of scleroderma. (A) Lethally irradiated B6D2F1 recipients were transplanted with 5 × 106 TCD BM cells with (BM+T) or without (TCD BM) 0.5 × 106 CD3+ T cells from B6.FoxP3-DTR-GFP donor mice. (B) After transplantation, the mice were injected intraperitoneally twice a week with saline or DT from day 7 for a period of 6 weeks. (C) Representative dot plots of CD4+FoxP3 GFP+ cells gated on CD3+CD4+ T cells in spleen after saline or DT treatment at day 28. The splenic cell number and the frequency and number of CD4+FoxP3 GFP+ cells within CD3+CD4+ T cells were analyzed in (D) spleen and (E) thymus at day 28 after BMT (n = 6-9). (F) Clinical scores and survival of the recipients from the BMT as described in A (**P < .01: BM+T-saline vs BM+T-cells-DT, ***P < .001: TCD BM-saline vs TCD BM-DT, 2 experiments). (G) Representative images of H&E staining of skin samples were taken at day 28 after BMT and quantified for GVHD histopathology (n = 6-9, 2 experiments).
Adoptive transfer of natural Treg cells attenuates cGVHD
Recently, a human study demonstrated that low-dose interleukin (IL)-2, required for homeostatic maintenance of Tregs, increases Tregs after BMT with consequent amelioration of cGVHD.32 Thus, therapeutic transfer and/or expansion of tTregs would appear to be a promising therapeutic approach for cGVHD. We therefore investigated whether the cGVHD that develops when CD4+FoxP3+ Tregs are absent could be prevented when naive donor CD4+ Tregs were adoptively transferred into these recipients. We depleted donor tTregs as previously described from day 7, and concurrently adoptively transferred sort purified CD4+ Treg from CD45.1+ congenic donors at day 14 (Figure 6A-B). Importantly, these adoptively transferred tTregs are not susceptible to depletion by DT as they do not express the DTR. The adoptive transfer of CD4+ Tregs resulted in significantly lower clinical scores and improved survival compared with the control mice (median survival DT: 40 days vs DT + Treg: 81 days, although most animals subsequently succumbed to GVHD; Figure 6C). Importantly, the adoptive transfer of CD4+ Tregs reduced skin pathology. In this regard, hematoxylin and eosin (H&E) and Masson’s trichrome staining (collagen fibers are stained blue) of skin harvested at D35 after transplant showed retention of subcutaneous fat and hair follicles and reduced fibrosis in BMT recipients receiving adoptively transferred CD4+ Tregs (Figure 6D). In conclusion, we showed that CD4+ Treg transfer significantly improved cutaneous cGVHD, confirming a critical role for CD4+ Tregs in the control of cGVHD.
Adoptive transfer of natural Tregs attenuates cGVHD. (A) Lethally irradiated C57BL/6 recipients were transplanted with 10 × 106 TCD BM cells with 3 × 106 CD3+ T cells from BALB/c.FoxP3-DTR-GFP donor mice. (B) After transplantation, the mice were injected intraperitoneally twice a week with DT from day 7. At day 14 after BMT, freshly sorted nTregs (CD4+CD25+) from BALB/c.CD45.1+ mice were adoptively transferred or not in the recipients. (C) Clinical scores and survival of the recipients (n = 16-34, 6 experiments). (D) Representative images of H&E and Masson’s trichrome staining of skin samples collected at day 35 after BMT. GVHD histopathology was quantified in the skin (n = 5, 1 experiment).
Adoptive transfer of natural Tregs attenuates cGVHD. (A) Lethally irradiated C57BL/6 recipients were transplanted with 10 × 106 TCD BM cells with 3 × 106 CD3+ T cells from BALB/c.FoxP3-DTR-GFP donor mice. (B) After transplantation, the mice were injected intraperitoneally twice a week with DT from day 7. At day 14 after BMT, freshly sorted nTregs (CD4+CD25+) from BALB/c.CD45.1+ mice were adoptively transferred or not in the recipients. (C) Clinical scores and survival of the recipients (n = 16-34, 6 experiments). (D) Representative images of H&E and Masson’s trichrome staining of skin samples collected at day 35 after BMT. GVHD histopathology was quantified in the skin (n = 5, 1 experiment).
Discussion
Over the last decade, cGVHD has become a significant clinical problem following allogeneic BMT, owing in part to the increased incidence resulting from the increased use of G-CSF PB as the stem cell source. Unfortunately, the pathophysiology of cGVHD is poorly understood, and greater mechanistic insights are required to allow the strategic development of specific treatment. Several studies have demonstrated an association between cGVHD and reduced circulating Treg numbers, implicating a role for this population in maintaining immune tolerance following allogeneic BMT.17-19 However, the factors contributing to reduced Treg numbers after transplant and a direct contribution of this Treg deficit to cGVHD have yet to be fully elucidated. In this study, we demonstrate that impaired MHC class II antigen presentation by cDCs after transplant results in a failure of Treg homeostasis and that this promotes the development of cGVHD, which could be ameliorated by the adoptive transfer of nTreg.
The single most effective clinical predictor for the development of cGVHD is the prior development of aGVHD, although cGVHD can occur in the absence of prior aGVHD. However, there remains the possibility that low-level alloreactive responses occur even in patients who do not meet diagnostic criteria for low-grade GVHD and that this subclinical aGVHD may contribute to the subsequent development of cGVHD. Thus, clarification of how aGVHD functions to promote cGVHD is required. In the current studies, we used a low dose of donor T cells in the BM graft to induce cGVHD pathology late after transplant, which is preceded by subtle aGVHD. In this setting, cGVHD was associated with diminished MHC class II antigen presentation within donor cDCs as we have previously reported in the setting of aGVHD.27 Our previous studies have explored the contribution of inflammatory pathways in cDC impairment, and although the GVHD-associated cytokine milieu was critical in the process, the only cytokine found to specifically contribute was tumor necrosis factor, and this was only partial. Factors such as glucocorticoids, IL-10, and transforming growth factor β have been reported to promote the differentiation of tolerogenic Treg-inducing DCs33,34 ; however, whether a deficiency in these signaling pathways occurs in GVHD or impacts on antigen presentation has yet to be explored. Importantly, we now demonstrate for the first time that the majority of donor DCs are presenting donor-derived antigen compared with host-derived alloantigen and that a quantitative impairment of both occurs during GVHD in the periphery.
Having demonstrated perturbed cDC MHC class II–restricted antigen presenting function in the setting of GVHD, and as cDCs are known to play a key role in the induction and maintenance of Tregs,21 we studied whether Treg deficiency after transplant is a direct consequence of defective cDC function. In support of a role for defective MHC class II antigen presentation in cGVHD pathology, a previous study reported that donor MHC class II deficiency leads to cGVHD due to a lack of MHC class II expression on thymic cDCs, resulting in impaired thymic selection and the development and release of pathogenic autoreactive T cells.9 Notably, in this study and ours, the number of Treg in the thymus was not, or only minimally affected. In contrast, in the absence of donor MHC class II, we found a striking reduction in Treg number in the periphery. Moreover, we demonstrate that MHC class II expression by donor cDCs specifically is required for Treg maintenance, the absence of which results in the development of cGVHD. In support of this, a recent study demonstrated that donor Treg maintenance after stem cell transplantation is promoted via donor, but not host, cDCs.23 Thus, we established a causative relationship between aGVHD, impaired antigen presentation by donor DCs, and altered Treg homeostasis culminating in the development of cGVHD.
Our data highlight the potential for adoptive Treg therapy to inhibit or reverse cGVHD after allogeneic BMT. Adoptive Treg therapy has been shown to be a safe and effective therapy in multiple mouse and humanized mouse models.16,35,36 Moreover, several adoptive Treg clinical trials are published37-40 and more are in the pipeline. Thus, this approach holds promise for inducing robust tolerance in clinical transplant patients. Indeed, our data showed a marked, albeit incomplete, improvement in clinical scores, survival, and cGVHD pathology following the adoptive transfer of nTregs. However, the failure to completely alleviate cGHVD highlights the need to develop improved Treg-based cell therapy strategies to treat cGVHD. In this regard, the significant factors limiting the application of this therapy include the scarcity of Tregs in human blood, inherent FoxP3, and functional instability of Tregs15,41 and their poor survival after transfer. Therefore, studies to date have based their effort on focusing on approaches to modulate nTreg expansion and/or stabilization. The therapeutic ratio of Tregs to conventional CD4 T cells can be as high as 10:1; thus, collection of sufficient numbers of unmanipulated donor Tregs from blood for adequate treatment doses is difficult. Strategies to overcome this barrier include ex vivo tTreg expansion and the in vitro generation of induced Tregs from conventional CD4 T cells, both of which can yield realistic therapeutic doses.42,43 Additionally, alternate immunosuppressive strategies to improve Treg survival after transplant are under investigation.44,45 IL-2 is a cytokine known to improve survival, proliferation, and function of T lymphocytes and also Tregs, as they express the high affinity IL-2 receptor (CD25). Encouragingly, in recent clinical studies, treatment of cGVHD patients with low-dose IL-2 elicited a reduction in cGVHD signs and symptoms in one half of the patients studied.32,46,47 The individual use or combination of the mechanistic target of rapamycin pathway inhibitor rapamycin with IL-2/anti–IL-2 antibody complexes has also improved Treg stability.15,48,49 However, further clinical studies are required to improve the expansion of nTregs after their transfer into patients.
In conclusion, we demonstrated that cGVHD is induced by a failure in CD4+FoxP3+ Tregs in the periphery, and not in the thymus, as a consequence of impaired MHC class II antigen presentation by donor cDCs after transplant induced by even subtle levels of aGVHD. Importantly, this study highlights the therapeutic potential for the adoptive transfer of CD4+FoxP3+ Tregs and/or the therapeutic expansion of CD4+FoxP3+ Tregs in vivo to treat cGVHD in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the MacDonald and Hill laboratories for helpful discussion and suggestions and the staff at the QIMR Berghofer Medical Research Institute animal facility and Grace Chojnowski, Paula Hall, Michael Rist, and Melinda Christensen from the QIMR Berghofer Medical Research Institute Flow Cytometry facility for assistance. I-Abflox/flox mice were a kind gift from P.A. Koni, Augusta University, USA.
L.L.-E.m. was supported by the University of Queensland International Research Tuition Award and the University of Queensland Research Scholarship. M.C. is supported by a Leukemia Foundation of Queensland scholarship. G.R.H. is an National Health and Medical Research Council (NHMRC) Australia Fellow and Queensland Health Senior Clinical Research Fellow. K.P.A.M. is a Cancer Council Queensland Senior Research Fellow. This work was funded by an NHMRC of Australia grant to K.P.A.M. (APP1031728) and National Institutes of Health grants from the National Heart, Lung, and Blood Institute (R01 HL11879) and National Institute of Allergy and Infectious Diseases (P01 AI-56299).
Authorship
Contribution: L.L.-E.m. designed and performed experiments and wrote the manuscript; M.K. designed and performed experiments and interpreted data; L.L.T. and K.A.M. designed and performed experiments; M.C., R.D.K., K.E.L., B.E.T., and K.A.A. performed experiments; A.D.C. performed blinded histologic assessment; B.R.B. contributed to experimental design and interpretation of data; G.R.H. and K.P.A.M. contributed to experimental design and interpretation of data and cowrote the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kelli P. A. MacDonald, QIMR Berghofer Medical Research Institute, 300 Herston Rd, Brisbane, QLD 4006, Australia; e-mail: kelli.macdonald@qimrberghofer.edu.au.
References
Author notes
L.L.-E.m. and M.K. contributed equally to this work.
G.R.H. and K.P.A.M. contributed equally to this work.