Key Points
rVIII-SingleChain is a novel rFVIII, designed to have high stability and high binding affinity for VWF.
In severe hemophilia A patients, rVIII-SingleChain was well tolerated and resulted in low bleeding rates, when dosed twice per week.
Abstract
Recombinant VIII (rVIII)-SingleChain is a novel B-domain–truncated recombinant factor VIII (rFVIII), comprised of covalently bonded factor VIII (FVIII) heavy and light chains. It was designed to have a higher binding affinity for von Willebrand factor (VWF). This phase 1/3 study investigated the efficacy and safety of rVIII-SingleChain in the treatment of bleeding episodes, routine prophylaxis, and surgical prophylaxis. Participants were ≥12 years of age, with severe hemophilia A (endogenous FVIII <1%). The participants were allocated by the investigator to receive rVIII-SingleChain in either an on-demand or prophylaxis regimen. Of the 175 patients meeting study eligibility criteria, 173 were treated with rVIII-SingleChain, prophylactically (N = 146) or on-demand (N = 27). The total cumulative exposure was 14 306 exposure days (EDs), with 120 participants reaching ≥50 EDs and 52 participants having ≥100 EDs. Hemostatic efficacy was rated by the investigator as excellent or good in 93.8% of the 835 bleeds treated and assessed. Across all prophylaxis regimens, the median annualized spontaneous bleeding rate was 0.00 (Q1, Q3: 0.0, 2.4) and the median overall annualized bleeding rate (ABR) was 1.14 (Q1, Q3: 0.0, 4.2). Surgical hemostasis was rated as excellent/good in 100% of major surgeries by the investigator. No participant developed FVIII inhibitors. In conclusion, rVIII-SingleChain is a novel rFVIII molecule showing excellent hemostatic efficacy in surgery and in the control of bleeding events, low ABR in patients on prophylaxis, and a favorable safety profile in this large clinical study. This trial was registered at www.clinicaltrials.gov as #NCT01486927.
Introduction
Hemophilia is an X-linked congenital bleeding disorder caused by a coagulation factor deficiency, which affects an estimated 1 in 10 000 births. The primary aim of care is to prevent and treat bleeding using coagulation factor replacement therapy.1 In hemophilia care today, challenging unmet needs remain to be addressed; among those are the poor uptake of prophylaxis, and the prevention of hemophilic arthropathy and inhibitor development.2 Optimization of prophylaxis to prevent (or delay) functional deterioration of an existing hemophilic arthropathy and development of less immunogenic replacement clotting concentrates are the potential solutions. Products with improved pharmacokinetics (PK) and innovative dosing regimens have the potential to reduce the frequency of injections with current prophylactic regimens, improve compliance, and reduce the burden of musculoskeletal complications of recurrent joint bleeds.3
The plasma half-life of most currently available factor VIII (FVIII) products means patients are required to inject FVIII every other day or 3 times a week, resulting in poor compliance.4,5 Recently, several new recombinant FVIII (rFVIII) products with extended half-life have completed phase 3 studies.6,7 Although (glycol) pegylation and Fc fusion have prolonged the half-life of rFVIII, this extension is limited to only 1.5 to 1.7 times the normal half-life of endogenous FVIII. This is largely due to the dependence of FVIII on the half-life of von Willebrand factor (VWF) in the circulation.8
Immunogenicity remains the other major challenge of replacement therapy in FVIII-deficient patients. Ex vivo studies suggest that in addition to protection from proteolysis, VWF prevents uptake of FVIII by antigen-presenting cells.9,10 This mechanism is presumed to mitigate the risk of inhibitor development; therefore, improved binding of FVIII to VWF may reduce the likelihood of inhibitor formation.
The recombinant VIII (rVIII)-SingleChain is comprised of the FVIII heavy and light chain covalently fused into a single polypeptide protein, which upon activation by thrombin, is indistinguishable from endogenous activated FVIII.11 The single-chain design results in a stable and homogenous drug product with increased binding affinity for VWF, and PK properties that are superior to those of full-length rFVIII.12 Of note, these favorable PK attributes were achieved without glycopegylation or fusion to antibody fragments.
Here, we report the efficacy, safety, and PK results of a prospective phase 1/3 study investigating rVIII-SingleChain for prophylaxis, on-demand treatment, and perioperative management of severe hemophilia A.
Methods
Study design and patients
This open-label, nonrandomized multicenter study recruited males with severe hemophilia A (FVIII activity <1%), previously treated with FVIII (>150 exposure days [EDs] prior to enrollment), and aged between 12 and 65 years. Patients with a personal or family history (first-degree relatives) of FVIII inhibitors, or a detectable inhibitor titer at screening were excluded from the study. Other exclusion criteria were laboratory evidence of hepatic and renal failure, and immunosuppression (including low CD4 counts in HIV-positive patients). For full inclusion and exclusion criteria see supplemental Methods, available on the Blood Web site.
The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the ethical principles outlined in the Declaration of Helsinki 2008.13 Ethics approval, individual informed consent, and approval by the relevant national authorities was obtained prior to enrollment. The safety of study participants was overseen by an Independent Data Monitoring Committee.
Dosing
Participants were assigned to either prophylaxis or on-demand therapy by the investigator and switching therapies was not permitted during the study. Patients on routine prophylaxis could be prescribed 20 to 40 IU/kg rVIII-SingleChain every second day or 20 to 50 IU/kg rVIII-SingleChain 2 to 3 times per week, or at other doses or dosing frequencies at the investigator’s discretion. The FVIII treatment regimen used prior to enrollment and the patient’s bleeding phenotype were taken into account. The prescription for dosing rVIII-SingleChain to treat bleeding in patients receiving on-demand therapy or breakthrough bleeds in patients on routine prophylaxis was guided by the World Federation of Hemophilia (WFH) recommendations for the treatment of different types of bleeding locations and intensity.14 The dose could be adjusted during the study if necessary.
For patients undergoing surgery, the rVIII-SingleChain dosing regimen was individualized, based on the type of surgery and the clinical status of the patient. The dosing was adjusted in the pre-, intra-, and postsurgical settings to achieve and maintain an FVIII activity level recommended by the WFH guidelines.1
Efficacy end points
The primary efficacy end points of this study were the annualized spontaneous bleeding rate (AsBR) and hemostatic efficacy in the control of bleeding episodes, and during surgery. The secondary efficacy end-points were the annualized bleeding rate (ABR) for all bleeds and the number of injections of rVIII-SingleChain required to achieve hemostasis. Bleeding episodes were either treated by the patient or, when in hospital, by the investigator, and classified as spontaneous when they occurred without apparent external cause, or traumatic when an injury preceded the bleeding event.
Hemostatic efficacy in bleeding events treated with rVIII-SingleChain was rated by the investigator on a 4-point rating scale, utilizing information provided by the patient and considering the number of doses needed to control the bleed (Table 1). The efficacy of rVIII-SingleChain in surgical prophylaxis was rated by the investigator on a 4-point rating scale, based on information from the surgeon on intraoperative hemostasis, and the anesthesia team on intraoperative blood loss and transfusion requirements (if any) (Table 1). Each treated bleed or surgery was assigned an efficacy rating of excellent, good, moderate, or no response. Treatments assigned an efficacy rating of excellent or good were considered a treatment success.
Investigator evaluation of hemostatic efficacy, 4-point scale
| Rating . | Treatment of bleeding events . | Treatment during surgery . |
|---|---|---|
| Excellent | Definite pain relief and/or improvement in signs of bleeding (ie, swelling, tenderness, and/or increased range of motion in the case of musculoskeletal hemorrhage) within ∼8 h after the first rVIII-SingleChain injection | Hemostasis clinically not significantly different from normal (eg, achieved hemostasis comparable to that expected during similar surgery in a nonfactor-deficient patient) in the absence of other hemostatic intervention, and estimated blood loss during surgery is not >20% higher than the predicted blood loss for the intended surgery |
| Good | Definite pain relief and/or improvement in signs of bleeding at ∼8 h after the first rVIII-SingleChain injection, but requires 2 injections for complete resolution | Normal or mildly abnormal hemostasis in terms of quantity and/or quality (eg, slight oozing, prolonged time to hemostasis with somewhat increased bleeding compared with a nonfactor-deficient patient in the absence of other hemostatic intervention), or estimated blood loss is >20% but ≤30% higher than the predicted blood loss for intended surgery |
| Moderate | Probable or slight beneficial effect within ∼8 h after the first rVIII-SingleChain injection; requires more than 2 injections for complete resolution | Moderately abnormal hemostasis in terms of quantity and/or quality (eg, moderate hemorrhage that is difficult to control) with estimated blood loss greater than what is defined as “good” |
| Poor/no response | No improvement at all or condition worsens (ie, signs of bleeding) after the first rVIII-SingleChain injection and additional hemostatic intervention is required with another FVIII product, cryoprecipitate, or plasma for complete resolution | Severely abnormal hemostasis in terms of quantity and/or quality (eg, severe hemorrhage that is difficult to control) and/or additional hemostatic intervention required with another FVIII product, cryoprecipitate, or plasma for complete resolution |
| Rating . | Treatment of bleeding events . | Treatment during surgery . |
|---|---|---|
| Excellent | Definite pain relief and/or improvement in signs of bleeding (ie, swelling, tenderness, and/or increased range of motion in the case of musculoskeletal hemorrhage) within ∼8 h after the first rVIII-SingleChain injection | Hemostasis clinically not significantly different from normal (eg, achieved hemostasis comparable to that expected during similar surgery in a nonfactor-deficient patient) in the absence of other hemostatic intervention, and estimated blood loss during surgery is not >20% higher than the predicted blood loss for the intended surgery |
| Good | Definite pain relief and/or improvement in signs of bleeding at ∼8 h after the first rVIII-SingleChain injection, but requires 2 injections for complete resolution | Normal or mildly abnormal hemostasis in terms of quantity and/or quality (eg, slight oozing, prolonged time to hemostasis with somewhat increased bleeding compared with a nonfactor-deficient patient in the absence of other hemostatic intervention), or estimated blood loss is >20% but ≤30% higher than the predicted blood loss for intended surgery |
| Moderate | Probable or slight beneficial effect within ∼8 h after the first rVIII-SingleChain injection; requires more than 2 injections for complete resolution | Moderately abnormal hemostasis in terms of quantity and/or quality (eg, moderate hemorrhage that is difficult to control) with estimated blood loss greater than what is defined as “good” |
| Poor/no response | No improvement at all or condition worsens (ie, signs of bleeding) after the first rVIII-SingleChain injection and additional hemostatic intervention is required with another FVIII product, cryoprecipitate, or plasma for complete resolution | Severely abnormal hemostasis in terms of quantity and/or quality (eg, severe hemorrhage that is difficult to control) and/or additional hemostatic intervention required with another FVIII product, cryoprecipitate, or plasma for complete resolution |
In participants assigned to a prophylaxis or on-demand regimen, the ABR was calculated for all bleeds and the AsBR for spontaneous bleeding events.
Safety end points
The primary safety end point was the rate of inhibitor formation to FVIII evaluated from the time of first dose through the end-of-study visit. Inhibitory antibodies against FVIII were determined using the Nijmegen-modified Bethesda assay, as previously described.14 Anti–rVIII-SingleChain antibodies were determined via a 2-tiered approach using direct-binding enzyme-linked immunosorbent assays. Antibodies against rFVIII-Chinese hamster ovary cell proteins were detected using a validated enzyme-linked immunosorbent assay and confirmed using Surface Plasmon Resonance technology (see supplemental Methods for more details).
Safety was further assessed on the basis of the following secondary end points: local tolerability at the site of injection assessed by investigator and participant; the number, type, and severity of adverse events (AEs); laboratory safety parameters (hematology and biochemistry); and vital signs and physical examination.
PK assessment
The potency of rVIII-SingleChain was assigned using the chromogenic substrate assay calibrated against the World Health Organization FVIII standard (see supplemental Methods for details). PK was assessed in a subgroup of patients following the initial dose (50 IU/kg ± 10%) and repeated dosing; pre-dose, 10 to 15 minutes, 0.5, 1, 3, 6, 9, 24, 28, 32, 48, 72, and 96 hours. A noncompartmental PK analysis of FVIII activity in plasma was performed, with and without baseline correction, for the individual participant plasma FVIII activity-vs-time data using Model 202 for constant injection in WinNonlin 6.3.0 (Phoenix Build 6.3.0.395; Pharsight Corp, St. Louis, MO). The following parameters were calculated: incremental recovery (IR), maximum observed FVIII activity (Cmax), time to Cmax, terminal half-life, clearance, volume of distribution at steady-state, area under the curve (time zero to last quantifiable FVIII activity and time zero extrapolated to infinity), and mean residence time.
Statistical analysis
Efficacy analyses were conducted in participants who received at least 1 dose of rVIII-SingleChain as part of either on-demand treatment or routine prophylaxis. The ABR and the AsBR were calculated according to the following formula: number of treated events × 365.25 / efficacy evaluation period, excluding data from the PK and surgical parts of the study. Descriptive statistics included the median and interquartile range. Data from all prophylaxis regimens was combined and compared with on-demand therapy. Statistical comparisons were based on estimates from a Poisson model. All tests were performed at the 2-sided 0.05 level of significance.
Safety was assessed in all participants exposed to rVIII-SingleChain. The study was sufficiently powered to rule out an estimated incidence of inhibitor development of more than 6.8%. An exact 2-sided 95% Clopper-Pearson confidence interval (CI) (or 1-sided 97.5% upper confidence limit) was to be used for estimating the incidence of inhibitor formation.
Results
Study population
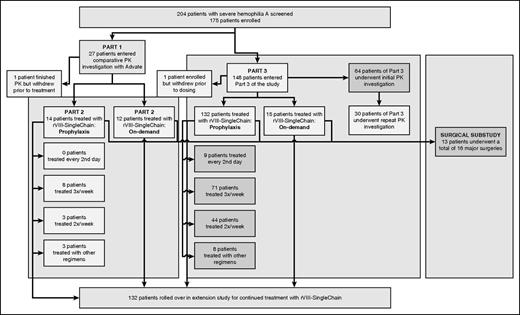
Participant disposition in the study is shown in Figure 1. Of the 204 patients screened, 175 met study eligibility criteria and were enrolled into the study. In total, 173 patients completed the study; 146 received prophylaxis and 27 received on-demand therapy. At screening, hemophilic arthropathy was reported by 15 patients (56%) assigned to on-demand therapy and by 71 patients (49%) assigned to prophylactic therapy.
The initial 27 patients who entered the study underwent a comparative PK investigation (Part 1), the results of which have been reported previously.12 Of the 27 patients who completed the PK investigation, 26 patients continued into a treatment phase (Part 2) and 1 patient elected to withdraw from the study. Participants were assigned to either prophylaxis (N = 14) or on-demand therapy with rVIII-SingleChain (N = 12).
Part 3 of the study recruited 148 additional patients and 1 patient withdrew prior to dosing with rVIII-SingleChain. Sixty-four of the 147 patients in Part 3 underwent an initial PK investigation. Of these, 30 patients underwent a repeat PK investigation 3- to 6-months later. As in Part 2, patients in Part 3 were assigned to either prophylaxis (N = 132) or on-demand therapy with rVIII-SingleChain (N = 15).
Thirteen patients underwent 16 surgical procedures during the surgical substudy. Overall, the study patients accumulated 14 306 EDs with rVIII-SingleChain; 120 patients were treated for ≥50 EDs, and of those, 52 received rVIII-SingleChain for ≥100 EDs. Demographics of the patients who received rVIII-SingleChain are shown in Table 2.
Participant demographics
| . | On-demand treatment arm (N = 27) . | Prophylaxis treatment arm (N = 146) . | Total study population (N = 174) . |
|---|---|---|---|
| Age (y), median (range) | 39.0 (23-64) | 28.0 (12-58) | 29.5 (12-64) |
| Age group, N (%) | |||
| ≥12 to <18 y | 0 | 14 (9.6) | 14 (8.0) |
| ≥18 to ≤65 y | 27 (100) | 132 (90.4) | 160 (92.0) |
| Weight (kg), mean (SD) | 78.1 (15.63) | 74.0 (17.26) | 74.6 (16.99) |
| BMI (kg/m2), mean (SD) | 25.2 (4.07) | 24.1 (4.82) | 24.3 (4.70) |
| Race, N (%) | |||
| Asian | 1 (3.7) | 30 (20.5) | 31 (17.8) |
| Black or African American | 3 (11.1) | 11 (7.5) | 14 (8.0) |
| White | 23 (85.2) | 102 (69.9) | 126 (72.4) |
| Other | 0 | 3 (2.1) | 3 (1.7) |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 2 (7.4) | 10 (6.8) | 12 (6.9) |
| Not Hispanic or Latino | 25 (92.6) | 135 (92.5) | 161 (92.5) |
| Not reported | 0 | 1 (0.7) | 1 (0.6) |
| Geographical region, N (%) | |||
| United States | 4 (14.8) | 18 (12.3) | 22 (12.6) |
| Japan | 1 (3.7) | 9 (6.2) | 10 (5.7) |
| Europe | 16 (59.3) | 69 (47.3) | 86 (49.4) |
| Rest of the world | 6 (22.2) | 50 (34.2) | 56 (32.2) |
| . | On-demand treatment arm (N = 27) . | Prophylaxis treatment arm (N = 146) . | Total study population (N = 174) . |
|---|---|---|---|
| Age (y), median (range) | 39.0 (23-64) | 28.0 (12-58) | 29.5 (12-64) |
| Age group, N (%) | |||
| ≥12 to <18 y | 0 | 14 (9.6) | 14 (8.0) |
| ≥18 to ≤65 y | 27 (100) | 132 (90.4) | 160 (92.0) |
| Weight (kg), mean (SD) | 78.1 (15.63) | 74.0 (17.26) | 74.6 (16.99) |
| BMI (kg/m2), mean (SD) | 25.2 (4.07) | 24.1 (4.82) | 24.3 (4.70) |
| Race, N (%) | |||
| Asian | 1 (3.7) | 30 (20.5) | 31 (17.8) |
| Black or African American | 3 (11.1) | 11 (7.5) | 14 (8.0) |
| White | 23 (85.2) | 102 (69.9) | 126 (72.4) |
| Other | 0 | 3 (2.1) | 3 (1.7) |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 2 (7.4) | 10 (6.8) | 12 (6.9) |
| Not Hispanic or Latino | 25 (92.6) | 135 (92.5) | 161 (92.5) |
| Not reported | 0 | 1 (0.7) | 1 (0.6) |
| Geographical region, N (%) | |||
| United States | 4 (14.8) | 18 (12.3) | 22 (12.6) |
| Japan | 1 (3.7) | 9 (6.2) | 10 (5.7) |
| Europe | 16 (59.3) | 69 (47.3) | 86 (49.4) |
| Rest of the world | 6 (22.2) | 50 (34.2) | 56 (32.2) |
BMI, body mass index; N, number of participants; SD, standard deviation.
rVIII-SingleChain in prophylaxis
In this study, patients received prophylaxis in a regimen that was assigned by the investigator, taking into account the patient’s FVIII treatment regimen used prior to enrollment and the patient’s bleeding phenotype. Of the 146 patients on prophylaxis, 79 (54%) were assigned a 3 times per week regimen, 47 (32%) a twice per week regimen, 9 (6%) an rVIII-SingleChain every other day regimen, and 11 (8%) were assigned other regimens (Figure 1). Data on the previous dosing regimen was available for 121 patients. Prior to enrollment, 73 patients (60%) were treated with an on-demand regimen and 48 patients (40%) were treated with prophylaxis. Treatment regimens prior to study entry and at the end of this study are shown in Table 3. A comparison of previous and end-of-study treatment regimens for the 48 patients in whom information on the previous treatment regimen was available and who were treated with prophylaxis therapy prior to enrollment are shown in Table 4.
Treatment regimens prior to study entry and at the end of this study
| . | Prior to study (N = 121) . | End of study (N = 121) . |
|---|---|---|
| Every 2nd day | 9 (7%) | 8 (7%) |
| 3 times weekly | 25 (21%) | 57 (47%) |
| 2 times weekly | 6 (5%) | 32 (26%) |
| Other regimen | 8 (7%) | 11 (9%) |
| On-demand | 73 (60%) | 13 (11%) |
| . | Prior to study (N = 121) . | End of study (N = 121) . |
|---|---|---|
| Every 2nd day | 9 (7%) | 8 (7%) |
| 3 times weekly | 25 (21%) | 57 (47%) |
| 2 times weekly | 6 (5%) | 32 (26%) |
| Other regimen | 8 (7%) | 11 (9%) |
| On-demand | 73 (60%) | 13 (11%) |
Comparison of previous and end-of-study treatment regimens for subjects treated with prophylaxis therapy prior to enrollment
| . | Prior to study (N = 48) . | End of study (N = 48) . |
|---|---|---|
| Every 2nd day | 9 (19%) | 4 (8%) |
| 3 times weekly | 25 (52%) | 18 (38%) |
| 2 times weekly | 6 (12%) | 17 (35%) |
| Other regimen | 8 (17%) | 9 (19%) |
| . | Prior to study (N = 48) . | End of study (N = 48) . |
|---|---|---|
| Every 2nd day | 9 (19%) | 4 (8%) |
| 3 times weekly | 25 (52%) | 18 (38%) |
| 2 times weekly | 6 (12%) | 17 (35%) |
| Other regimen | 8 (17%) | 9 (19%) |
Patients on prophylaxis 3 times per week were assigned doses by their individual investigator; median dose was 30 IU/kg per injection. Patients assigned a twice per week regimen used a median dose of 35 IU/kg. The median consumption of rVIII-SingleChain across all prophylaxis regimens was 4283 IU/kg per year (mean ± SD, 4494 ± 1778.17 IU/kg) (Table 5).
Dosing and consumption of rVIII-SingleChain, AsBR, ABR, and location of bleeds with rVIII-SingleChain in on-demand therapy and prophylaxis
| . | On-demand (N = 27) . | Prophylaxis . | ||
|---|---|---|---|---|
| All (N = 146) . | Three times per week (N = 79) . | Twice per week (N = 47) . | ||
| Dose, IU/kg | ||||
| Median (Q1, Q3) | 30 (25, 40) | 31 (27, 40) | 30 (26, 38) | 35 (30, 41) |
| Consumption, IU/kg per year | ||||
| Median | — | 4283 | 4514 | 3669 |
| Mean (SD) | — | 4494 (1778.17) | 4769 (1237.42) | 3974 (2396.93) |
| AsBR | ||||
| Median (Q1, Q3) | 11.73 (2.8, 36.5) | 0.0 (0.0, 2.4) | 0.0 (0.0, 3.6) | 0.0 (0.0, 1.1) |
| Mean (SD) | 24.84 (33.84) | 2.10 (4.76) | 2.33 (3.87) | 2.33 (6.67) |
| N bleeds per year* (95% CI) | 19.5 (17.8-21.3) | 1.6 (1.3-1.8) | 1.9 (1.6-2.3) | 1.3 (1.0-1.8) |
| ABR | ||||
| Median (Q1, Q3) | 19.64 (6.2, 46.5) | 1.14 (0.0, 4.2) | 1.93 (0.0, 4.9) | 0.0 (0.0, 3.3) |
| Mean (SD) | 31.14 (35.56) | 3.11 (5.05) | 3.34 (4.26) | 3.27 (6.83) |
| N bleeds per year† (95% CI) | 24.9 (23.0-27.0) | 2.6 (2.3-2.9) | 2.9 (2.5-3.4) | 2.4 (1.9-3.0) |
| Location of spontaneous bleeds, N (%) | ||||
| Joint | 419 (91.1) | 147 (94.2) | 104 (97.2) | 39 (95.1) |
| Muscle | 55 (12.0) | 11 (7.1) | 7 (6.5) | 3 (7.3) |
| Other | 57 (12.4) | 12 (7.7) | 8 (7.5) | 1 (2.4) |
| . | On-demand (N = 27) . | Prophylaxis . | ||
|---|---|---|---|---|
| All (N = 146) . | Three times per week (N = 79) . | Twice per week (N = 47) . | ||
| Dose, IU/kg | ||||
| Median (Q1, Q3) | 30 (25, 40) | 31 (27, 40) | 30 (26, 38) | 35 (30, 41) |
| Consumption, IU/kg per year | ||||
| Median | — | 4283 | 4514 | 3669 |
| Mean (SD) | — | 4494 (1778.17) | 4769 (1237.42) | 3974 (2396.93) |
| AsBR | ||||
| Median (Q1, Q3) | 11.73 (2.8, 36.5) | 0.0 (0.0, 2.4) | 0.0 (0.0, 3.6) | 0.0 (0.0, 1.1) |
| Mean (SD) | 24.84 (33.84) | 2.10 (4.76) | 2.33 (3.87) | 2.33 (6.67) |
| N bleeds per year* (95% CI) | 19.5 (17.8-21.3) | 1.6 (1.3-1.8) | 1.9 (1.6-2.3) | 1.3 (1.0-1.8) |
| ABR | ||||
| Median (Q1, Q3) | 19.64 (6.2, 46.5) | 1.14 (0.0, 4.2) | 1.93 (0.0, 4.9) | 0.0 (0.0, 3.3) |
| Mean (SD) | 31.14 (35.56) | 3.11 (5.05) | 3.34 (4.26) | 3.27 (6.83) |
| N bleeds per year† (95% CI) | 24.9 (23.0-27.0) | 2.6 (2.3-2.9) | 2.9 (2.5-3.4) | 2.4 (1.9-3.0) |
| Location of spontaneous bleeds, N (%) | ||||
| Joint | 419 (91.1) | 147 (94.2) | 104 (97.2) | 39 (95.1) |
| Muscle | 55 (12.0) | 11 (7.1) | 7 (6.5) | 3 (7.3) |
| Other | 57 (12.4) | 12 (7.7) | 8 (7.5) | 1 (2.4) |
Q1, lower quartile; Q3, upper quartile.
Estimated number of spontaneous bleeds per subject per year based on a Poisson distribution.
Estimated number of bleeds per subject per year based on a Poisson distribution.
Across all prophylaxis regimens, the observed median AsBR was 0.0 (Q1, Q3: 0.0, 2.4) and the observed mean ± SD AsBR was 2.1 ± 4.76. The calculated AsBR across all prophylaxis regimens was 1.6 bleeds per year (95% CI, 1.3-1.8). In the subgroup of patients who received prophylaxis twice a week, the observed median AsBR was 0.0 (Q1, Q3: 0.0, 1.1) and the observed mean ± SD AsBR was 2.33 ± 6.67, which were comparable with values in subjects who received prophylaxis 3 times a week (median AsBR, 0.0 [Q1, Q3: 0.0, 3.6] and mean ± SD AsBR, 2.33 ± 3.87). The AsBR with prophylaxis was markedly reduced (P < .0001) compared with the on-demand treatment group (median AsBR, 11.73 [2.8, 36.5]) (Table 5). The majority of spontaneous bleeding episodes that required treatment were located in the joint (Table 5).
The observed median ABR across all prophylaxis regimens was 1.14 (Q1, Q3: 0.0, 4.2) and the observed mean ± SD ABR was 3.11 ± 5.05. The calculated ABR across all prophylaxis regimens was 2.6 bleeds per year (95% CI, 2.3-2.9). In the subgroup of patients who received prophylaxis twice a week, the median observed ABR was 0.0 (Q1, Q3: 0.0, 3.3) and the observed mean ± SD ABR was 3.27 ± 6.83. These are not increased relative to patients who received rVIII-SingleChain 3 times week, in whom the observed median ABR was 1.93 (Q1, Q3: 0.0, 4.9) and the observed mean ± SD ABR was 3.34 ± 4.26. The ABR with prophylaxis was highly significantly (P < .0001) reduced compared with the ABR in the on-demand group (median ABR, 19.64 [Q1, Q3: 6.2, 46.5]) (Table 5).
rVIII-SingleChain in the control of bleeding events
A total of 848 bleeding events were treated with rVIII-SingleChain in the study, 835 of which were assessed by the investigator. Of the 848 bleeding events, 590 occurred in the 27 patients on on-demand therapy and 258 occurred in the 146 patients on prophylaxis. Of the patients on prophylaxis, 43% had no treated bleeds during the study. Of the 835 bleeding events assessed by the investigator, efficacy of rVIII-SingleChain to control the bleed was rated as excellent in 603 (72.2%), good in 180 (21.6%), and moderate in 52 (6.2%). No bleeds were reported as having a poor or no response to rVIII-SingleChain (Table 6).
Efficacy in the control of bleeding episodes and in prophylaxis
| . | N (%) . | Median dose (IU/kg) . |
|---|---|---|
| Bleeding events treated with rVIII-SingleChain | 848 | 34.7 |
| Bleeding events with investigator assessment | 835 (100) | 34.7 |
| Efficacy rating | ||
| Excellent | 603 (72.2) | 32.2 |
| Good | 180 (21.6) | 43.2 |
| Moderate | 52 (6.2) | 93.4 |
| Poor/no response | 0 (0) | N/A |
| Number of injections required to treat | ||
| 1 | 686 (80.9) | 31.2* |
| 2 | 107 (12.6) | 35.6* |
| ≥3 | 55 (6.5) | 37.6* |
| . | N (%) . | Median dose (IU/kg) . |
|---|---|---|
| Bleeding events treated with rVIII-SingleChain | 848 | 34.7 |
| Bleeding events with investigator assessment | 835 (100) | 34.7 |
| Efficacy rating | ||
| Excellent | 603 (72.2) | 32.2 |
| Good | 180 (21.6) | 43.2 |
| Moderate | 52 (6.2) | 93.4 |
| Poor/no response | 0 (0) | N/A |
| Number of injections required to treat | ||
| 1 | 686 (80.9) | 31.2* |
| 2 | 107 (12.6) | 35.6* |
| ≥3 | 55 (6.5) | 37.6* |
N/A, not applicable.
IU/kg for first injection.
For 80.9% of the bleeding events, patients required a single dose of rVIII-SingleChain to achieve hemostatic control. A second dose was required to achieve hemostatic control in 12.6% of bleeding events and 3 or more doses were required for 6.5% of bleeding events. The median cumulative dose used to treat a bleeding event was 34.7 IU/kg (mean, 45.4 IU/kg) and the median dose per injection to treat a bleeding event was 31.7 IU/kg (mean, 32.0 IU/kg). The doses used to treat bleeds that achieved hemostatic control after a single dose were not higher than the initial doses used to treat bleeds that required multiple doses for hemostatic control (Table 6).
rVIII-SingleChain in surgical prophylaxis
Thirteen patients underwent a total of 16 surgical procedures that required general, spinal, or regional anesthesia (wisdom teeth extraction [1], abdominal hernia repair [1], elbow replacement [1], ankle arthroplasty [1], knee replacement [5], cholecystectomy [1] combined with lengthening of the Achilles tendon and straightening of the right toes [1], circumcision [3], open reduction internal fixation right ankle [1], and hardware removal right ankle [1]). Two of these procedures (cholecystectomy combined with lengthening of the Achilles tendon and straightening of the right toes) were performed in the same session but received differentiated assessment of hemostasis. Overall, investigators’ assessed hemostatic efficacy of rVIII-SingleChain in surgical prophylaxis as excellent in 15/16 surgeries and as good in 1/16 surgeries. Median rVIII-SingleChain consumption (pre- and intraoperatively) was 89.36 IU/kg (range, 40.45-108.58 IU/kg).
Safety
Immunogenicity.
Safety was assessed in all 174 patients exposed to rVIII-SingleChain. FVIII inhibitors were not detected in any study patients; the inhibitor incidence was 0% (95% CI, 0.0-2.1). In the 120 patients with ≥50 EDs, the inhibitor incidence was 0% (95% CI, 0.0-3.0).
Eight patients entered the study with a positive test for non-inhibitory antidrug antibodies (ADAs) (ie, anti-FVIII immunoglobulin G [IgG] and/or IgM antibodies), prior to dosing with rVIII-SingleChain. Seven of these patients remained positive until the end-of-study/last-visit on-study and 1 patient, who started the study with positive IgG antibodies, became negative by the end-of-study visit. Four other patients became positive for IgG and/or IgM during the study; 2 patients had negative and 2 had positive antibody results at the end-of-study visit.
No patient had preexisting anti-Chinese hamster ovary cell protein antibodies or developed them during the study.
Tolerability.
rVIII-SingleChain was well tolerated. Of 13 580 injections in which tolerability was assessed by the patients, 99.3% reported no reactions, 0.5% very slight, 0.15% slight, 0.05% moderate, and none had severe reactions. Consistent with these patient assessments, for 552 (99.8%) of the investigator-assessed injections, the assessment of the reaction was “none.” Only 1 patient (0.2%) with erythema was assessed by the investigator as having a very slight or “barely perceptible” reaction.
AEs.
Of the 174 patients, 113 (64.9%) experienced a total of 292 treatment-emergent AEs and the majority (77%) were mild in severity (supplemental Table 1). Only 7.5% of subjects experienced AEs that were considered to be related to the study drug (N = 13 events). A single study drug-related AE, a case of hypersensitivity, was considered severe, and all others were mild or moderate in intensity. No patient withdrew from the study due to an AE. The 3 most common AEs reported in the study were nasopharyngitis, arthralgia, and headache.
Of the 10 serious AEs (SAEs) reported in this study, none led to withdrawal from the study and 1 was judged to be related by the investigator; this was an event of hypersensitivity for which the investigator hospitalized the patient for observation, administered steroids and antihistamines, which provided relief within 30 minutes and allowed for hospital discharge later on the same day. The patient remained on rVIII-SingleChain treatment and tolerated it well. No clinically evident thromboembolic events were observed during the study.
Evaluation of the FVIII PK following repeat IV administration of rVIII-SingleChain
The PK investigation in Part 3 of the study confirmed the rVIII-SingleChain PK properties of Part 1 as published previously.12 PK parameters after initial (after the first dose, N = 64) and repeat dosing (3- to 6-months later, N = 30) in Part 3 are summarized in Table 7. Because rVIII-SingleChain FVIII plasma activity is underestimated by the 1-stage clotting assay, PK parameters were based on plasma FVIII activity measured by the chromogenic substrate assay. Results demonstrated a stable (time-independent) PK profile with a half-life of 12.9 hours, IR of 1.99 IU/dL per IU/kg, and clearance of 3.05 mL/kg per hour after repeat dosing. The PK of adolescents (≥12 to <18 years) was not different from that of adults (≥18 years of age) (data not shown).
PK parameters following the first dose and following 3- to 6-months of treatment (repeat PK in Part 3)
| . | Mean (CV %) (N = 64) . | ||
|---|---|---|---|
| Parameter (unit) . | Initial PK total (N = 64) . | Repeat PK (N = 30) . | |
| Initial . | Repeat . | ||
| IR,*† (IU/dL)/(IU/kg) | 1.85 (21.8) | 1.90 (21.0) | 1.99 (17.7) |
| Cmax,*† IU/dL | 99.9 (19.9) | 103 (19.3) | 108 (17.2) |
| AUCt, IU·h/dL | 1780 (34.5) | 1783 (33.3) | 1850 (33.0) |
| AUCinf, IU·h/dL | 1830 (34.9) | 1840 (33.9) | 1880 (34.5) |
| CL, mL/kg, h | 3.15 (38.2) | 3.13 (32.6) | 3.05 (36.0) |
| Vss, mL/kg | 59.5 (23.9) | 60.3 (22.2) | 53.1 (16.4) |
| t1/2, h | 14.1 (27.1) | 14.2 (29.0) | 12.9 (29.4) |
| MRT, h | 20.3 (26.4) | 20.2 (27.8) | 18.9 (28.5) |
| . | Mean (CV %) (N = 64) . | ||
|---|---|---|---|
| Parameter (unit) . | Initial PK total (N = 64) . | Repeat PK (N = 30) . | |
| Initial . | Repeat . | ||
| IR,*† (IU/dL)/(IU/kg) | 1.85 (21.8) | 1.90 (21.0) | 1.99 (17.7) |
| Cmax,*† IU/dL | 99.9 (19.9) | 103 (19.3) | 108 (17.2) |
| AUCt, IU·h/dL | 1780 (34.5) | 1783 (33.3) | 1850 (33.0) |
| AUCinf, IU·h/dL | 1830 (34.9) | 1840 (33.9) | 1880 (34.5) |
| CL, mL/kg, h | 3.15 (38.2) | 3.13 (32.6) | 3.05 (36.0) |
| Vss, mL/kg | 59.5 (23.9) | 60.3 (22.2) | 53.1 (16.4) |
| t1/2, h | 14.1 (27.1) | 14.2 (29.0) | 12.9 (29.4) |
| MRT, h | 20.3 (26.4) | 20.2 (27.8) | 18.9 (28.5) |
Median (range) age of the 64 subjects included in the PK analysis is 26 (12-58) years. The CV represents the percentage of variability in each parameter and is calculated as the SD divided by the mean.
AUCinf, area under the curve extrapolated to infinity; AUCt, area under the curve to the last sample with quantifiable drug concentration; CL, clearance; CV, coefficient of variation; MRT, mean residence time; t1/2, terminal half-life; Vss, volume of distribution at steady state.
For IR and Cmax, the total number of participants with available pre-dose–corrected measurements was N = 63 at the initial PK assessment, and N = 29 at the repeat PK assessment.
For IR and Cmax, pre-dose correction was performed by subtracting each participant’s FVIII activity level before dosing from the activity level obtained at each time point after dosing. All other parameters are pre-dose uncorrected.
Discussion
In this large phase 1/3 clinical study of individuals aged 12 to 65 years with severe hemophilia A, rVIII-SingleChain demonstrated excellent efficacy for prophylactic treatment with 43% of patients having no treated bleeds during the study. The median annualized spontaneous bleed rates were 0.0 when administered either twice or 3 times per week. rVIII-SingleChain showed excellent efficacy in controlling bleeding episodes at doses that are in line with the WFH recommendations. Treatment success (ie, investigator rating of excellent or good) was documented in 93.8% of all bleeds assessed. Thirteen patients underwent 16 surgeries, including 7 joint surgeries/replacements with surgical hemostasis, which were rated excellent in 15 (94%) procedures and good in 1 (6%).
Across all prophylaxis regimens, a very low median ABR (1.14 [Q1, Q3: 0.0, 4.2]) and a median AsBR of 0.0 (Q1, Q3: 0.00, 2.4) was achieved. As common practice in pivotal registration studies for new FVIII products, this study had no comparator arm to allow a direct comparison of efficacy with other products. Although indirect comparisons across studies are limited and do not allow for superiority claims, all of the recently published studies follow the same regulatory guidance (European Medicines Agency 2011) and enroll similar populations (ie, patients with severe hemophilia A). Efmoroctocog alfa (Eloctate) reported a median ABR of 1.6 with individualized prophylaxis.7 Median ABR with turoctocog alfa (Novoeight) was 3.7 in adults and adolescents with 83% of patients receiving a 3 times per week prophylaxis schedule.15 Simoctocog alfa (Nuwiq) reported a median ABR of 0.9 in a small study of 32 adult patients; however, this bleeding rate was achieved with a prophylactic regimen requiring dosing every second day.16
This study was designed to reflect clinical practice and as such, patients were assigned to prophylaxis regimens based on the clinical judgment of the treating physician. This resulted in one-third of the study population being assigned to a 2 times weekly prophylaxis regimen and half of the population being assigned to a 3 times weekly regimen. ABRs in both populations were low (0 and 1.93, respectively), confirming the possibility of almost complete freedom of bleeding events when rVIII-SingleChain is prescribed with accurate clinical judgment.
The hemostatic efficacy reported here for rVIII-SingleChain (93.8% of bleeds rated as good or excellent) is comparable to that of other rFVIII products, which range from 81% to 96.1% of bleeds being rated as excellent or good.6,15,17,18 rVIII-SingleChain was also analogous in terms of the numbers of injections needed to achieve hemostatic control; 93.5% of bleeds were controlled with 1 or 2 injections. With 1 or 2 injections, efmoroctocog alfa controlled 97.7% of bleeding episodes, moroctocog alfa (Refacto) controlled 88% (of 677 bleeding events across 2 studies), and rurioctacog alfa pegol (Adynovate) controlled 95.9% of bleeding episodes.6,7,19
Of the patients on prophylaxis with rVIII-SingleChain, 43% achieved a zero bleed rate during the study. This is comparable to individualized prophylaxis with efmoroctocog alfa (45%) and twice-weekly prophylaxis with rurioctacog alfa pegol (39.6%).6,7
Treatment with rVIII-SingleChain during all surgical procedures resulted in very good control of bleeding, with hemostatic efficacy rated as excellent in 94% of procedures. These results are in line with those reported recently for other novel rFVIII products. Efmoroctocog alfa reported excellent hemostasis in 88% and good hemostasis in 12% of surgeries (N = 8). For turoctocog alfa, intraoperative hemostasis was reported as excellent in 62% and good in 38% of 13 patients undergoing 15 procedures.7,20
Dosing in our study was at the investigators’ discretion with a recommendation to start prophylaxis with a dose between 20 to 40 IU/kg rVIII-SingleChain every second day or 20 to 50 IU/kg 2 to 3 times per week. Other schedules could be prescribed at the investigators’ discretion. Six-percent of patients were dosed every second day, 54% were dosed 3 times per week (median dose, 30 IU/kg), 32% were dosed twice per week (median dose, 35 IU/kg), and 8% followed other dosing regimens. The adherence to the prophylaxis regimen was high, with 92.5% of patients receiving not <80% and not >120% of the number of doses prescribed by the investigator.
For the treatment of bleeding events, investigators were instructed to follow the dosing guidelines for different bleeding types as recommended by the WFH in the 2012 edition of the WFH guidelines.1 With these dosing instructions, the median dose per injection to treat a bleeding episode was 31.7 IU/kg (range, 6-84), which is in line with doses used with other new rFVIII products; 27.35 IU/kg per dose for efmoroctocog alfa and a mean dose of 45.6 IU/kg for turoctocog alfa was used to stop a bleed.7,15
The overall median consumption for subjects on rVIII-SingleChain prophylaxis was 4283 IU/kg per year, which is comparable to that of the recently approved long-acting efmoroctocog alfa where a median consumption of 4118.4 IU/kg per year was observed in a study investigating individualized regimens.21
To our knowledge, this pivotal study accumulated the most EDs for a new rFVIII product, with a total of 14 306 EDs in 174 patients. Of these, 120 patients were treated for ≥50 EDs and 52 for ≥100 EDs. Despite this significant exposure and a wide global reach with multiple ethnicities included in patient recruitment, no inhibitor development was observed in this study. The immunogenicity of this novel rFVIII molecule will be further explored in an ongoing study, including previously untreated patients (#NCT02172950).
Four patients became positive for non-inhibitory IgG ADAs during the study. At end of study visit, 2 of these patients had a negative antibody result and 2 patients remained positive. This rate of ADA development is in line with other recently approved rFVIII products,7 and the incidence of non-neutralizing antibodies against FVIII in healthy individuals and in patients with hemophilia.22,23 Eight patients had non-inhibitory ADAs prior to dosing with rVIII-SingleChain, 7 of whom remained ADA positive at the end of the study.
rVIII-SingleChain was well tolerated and showed a favorable AE/SAE profile similar to that described for other products of the same class. The 3 most common AEs reported were nasopharyngitis, arthralgia, and headache. Of the 10 SAEs reported in this large study, only 1 was judged to be related to the study drug by the investigator. This was an event of hypersensitivity that was controlled by the administration of steroids and antihistamines, allowing hospital discharge of the patient on the day of the event. The patient continued in the study. No participants discontinued due to AEs.
The increased binding affinity of rVIII-SingleChain for VWF translates into a favorable PK profile of rVIII-SingleChain when compared with octocog alfa (Advate).12 The previously reported PK parameters were confirmed in this study and remained consistent after repeated dosing.
In conclusion, this study, which was designed to reflect clinical practice, demonstrated with a robust data set that rVIII-SingleChain is highly efficacious in the treatment of bleeding events, routine prophylaxis, and in controlling hemostasis in a variety of surgical procedures in adolescents and adults with severe hemophilia A. The study also demonstrated that rVIII-SingleChain has a favorable safety profile and is well tolerated. Very low ABRs in patients on individualized prophylaxis hopefully has the potential to translate into prolonged freedom from debilitating joint disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Editorial assistance with the preparation of this manuscript was provided by Meridian HealthComms Ltd, funded by CSL Behring.
CSL Behring, Marburg, Germany, funded this study.
Authorship
Contribution: D.B.-K., N.B., T.L., A.V., K.S.L., and I.P. contributed to the design of the study, analysis and interpretation of data, drafted the manuscript, and reviewed and approved the final version; J.M., K.K., F.A.K., O.S., M.V.K., L.M.L., A.S., L.N.B., R.K., J.O., A.H., E.S., R.I.B., K.F., J.C.G., S.P., P.C., M.A.E., C.D.K., and L.R. contributed to acquisition and interpretation of data, revised the manuscript, and reviewed and approved the final version; and all authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Thanks to the AFFINITY Investigators for their participation in this study.
Conflict-of-interest disclosure: J.M. received research grants from Bayer, Biogen, CSL Behring, Novo Nordisk, and Roche. L.N.B. received research support from Bayer, Biogen, Baxalta, Green Cross Corporation, Pfizer, Opko Health, Novo Nordisk, and OctaPharma. R.K. received honoraria and research funding from Baxter, Bayer, Biogen, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, and Sobi. J.O. received reimbursement for attending symposia/congresses and/or honoraria for speaking or consulting, and/or funds for research from Bayer HealthCare, Baxter, Biogen, Biotest, CSL Behring, Grifols, Novo Nordisk, Octapharma, Sobi, and Pfizer. E.S. received fees as a speaker in meetings organized by Kedrion, acted as a paid consultant to Bayer, Pfizer, CSL Behring, Novo Nordisk, Grifols, Baxter, Baxalta, Biogen, Sobi, Octapharma, and Roche, and received an unrestricted research grant from Pfizer. R.I.B. received funding for clinical trials from Biogen, Boehringer Ingelheim, Bayer, Baxter, Pfizer, Daiichi Sankyo, Portola Pharmaceuticals, Astellas, and CSL Behring; participated in clinical advisory boards for Amgen, Biogen, Baxalta, Boehringer Ingelheim, Bayer, Alexion Pharmaceuticals, and Pfizer; received research support from Baxter, Bayer, Bristol-Myers Squibb, and Alexion Pharmaceuticals; and received conference travel support from Amgen, Novo Nordisk, Baxter, and Alexion Pharmaceuticals. K.F. received speaker fees from Bayer, Baxter, CSL Behring, Pfizer, and Novo Nordisk; performed consultancy for Bayer, Baxter, Biogen, CSL Behring, Novo Nordisk, and Pfizer; and received research support from Bayer, Wyeth/Pfizer, Baxter, and Novo Nordisk. J.C.G. served on advisory committees for Baxalta, Bayer, and CSL Behring and received research support from Baxalta. P.C. received honoraria from Bayer, Baxter, Biogen, CSL Behring, Novo Nordisk, Pfizer, and Sobi; served on advisory boards for Baxter, Biogen, CSL Behring, Novo Nordisk, Pfizer, and Sobi; and received research funding from CSL Behring, Novo Nordisk, and Pfizer. M.A.E. served as an advisory board participant, study investigator, and/or consultant for Baxalta, Biogen, Bio Products Laboratory, Kedrion, Novo Nordisk, Bayer, and Pfizer. I.P. received a research grant from CSL Behring; and received honoraria for occasional lectures and advisory board sessions from CSL Behring, Novo Nordisk, Biotest, and Pfizer. D.B.-K., N.B., T.L., A.V., and K.S.L. are employees of CSL Behring. The remaining authors declare no competing financial interests.
A list of additional members of the AFFINITY Investigator group appears in the supplemental Data.
Correspondence: Ingrid Pabinger, Clinical Division of Haematology and Haemostaseology, Medical Clinic I, Medical University Vienna, Waehringer Guertel 18-20, Vienna 1090, Austria; e-mail: ingrid.pabinger@meduniwien.ac.at.