Abstract
Platelets have multiple functions beyond their roles in thrombosis and hemostasis. Platelets support liver regeneration, which is required after partial hepatectomy and acute or chronic liver injury. Although it is widely assumed that platelets stimulate liver regeneration by local excretion of mitogens stored within platelet granules, definitive evidence for this is lacking, and alternative mechanisms deserve consideration. In-depth knowledge of mechanisms of platelet-mediated liver regeneration may lead to new therapeutic strategies to treat patients with failing regenerative responses.
Liver regeneration
The liver has a unique regenerative capacity.1 Full restoration of functional liver mass can occur following injury or surgical removal of part of the liver. The ultimate regenerative response of the liver occurs after a partial liver resection, during which up to 70% of liver tissue can be safely removed.2 The liver not only regenerates following physical removal of liver tissue, but regenerative responses occur also when liver tissue is damaged by (for example) toxins or viruses or by ischemia/reperfusion injury. Liver regeneration enables surgical procedures such as partial liver resections and living donor liver transplantation, in which liver regeneration is required in both the donor and recipient. Liver regeneration may also lead to spontaneous recovery of patients with acute liver failure, showing little or no evidence of histologic abnormalities within months after the disease. In addition, animal experiments and biopsy studies in humans have demonstrated that moderate fibrosis can resolve when the trigger is removed or when successful treatment is given, suggesting that the liver can even regenerate from chronic insults.3,4
Nevertheless, in part of the patients, liver regeneration is insufficient or the regenerative process is not initiated at all. Failing liver regeneration is frequent in patients with acute liver failure, or in patients who underwent extensive partial liver resections. For these patients, little therapeutic options are available. Those patients require a, sometimes emergent, liver transplantation or die of liver insufficiency.
Evidence for a stimulatory role of platelets in liver regeneration
Platelets are well known for their role in cessation of bleeding and inducing thrombosis. Recent studies show that platelets have many functions beyond physiologic or pathologic thrombus formation. They play a role in inflammation,5,6 are effectors of injury in a variety of pulmonary disorders and syndromes,7 facilitate tissue repair,8 and act in the growth and development of metastases of various cancers.9
It has been well established that platelets are vital for liver regeneration after a partial liver resection in animals.10-15 Significantly, liver regeneration after partial liver resection was shown to be substantially delayed in mice that were treated with drugs that inhibit platelet function or in mice with thrombocytopenia induced by chemotherapeutic drugs or platelet-depleting antibodies.10 Conversely, mice in which the platelet count was increased by treatment with thrombopoietin receptor agonists or by administration of platelet concentrates showed accelerated liver regeneration.13-15 In addition to the role of platelets in regeneration following a partial hepatectomy, it has been established that platelets delay fibrosis in animal models of chronic intoxication16,17 and that platelets facilitate repair after ischemia/reperfusion injury.18 However, studies have also identified detrimental effects of platelets following these insults.19
In humans, a low platelet count following partial hepatectomy or living donor liver transplantation has been shown to be a strong and independent predictor of postoperative liver dysfunction and postoperative mortality.20-22 In addition, it has been recently demonstrated that platelet transfusion in living donor transplant recipients is associated with better liver regeneration, as assessed by graft volume measurements by computed tomography.23 In the same study it was demonstrated that in those patients that do not receive intraoperative platelets transfusions, the intraoperative platelet count is positively associated with graft regeneration. Moreover, a small uncontrolled study in humans suggested that platelet transfusion improves liver function in patients with established cirrhosis.24
Controversies and uncertainties
Although substantial evidence from animal models and clinical studies support the pivotal role of platelets in liver regeneration, no consensus on the mechanism exists. It is generally assumed that platelets support liver regeneration by delivery of mitogenic factors to the liver remnant of injured liver.25-28 Indeed, platelets rapidly accumulate within the liver sinusoids following a partial liver resection in animal models and occasionally are found in the Space of Disse or even within hepatocytes.11,29 It has been well established that aged or dysfunctional platelets are cleared from circulation by hepatocytes as a result of an interaction between desialylated platelet glycoprotein Ibα and the hepatocyte Ashwell-Morell receptor.30-32 In vitro studies, however, have shown that platelet uptake by hepatocytes in the context of platelet-mediated liver regeneration proceeds in an Ashwell-Morell receptor-independent manner.29 Platelets have also been shown to accumulate in the liver of humans within 1.5 hours after partial liver resection33 and appear deposited in livers from patients with cirrhosis.34,35 It is conceivable that this rapid platelet accumulation results in excretion of growth factors stored within platelet granules resulting in enhancement of the regenerative response. Platelets store hepatocyte growth factor (HGF), insulin-like growth factor (IGF), and serotonin that have been shown to stimulate hepatocyte proliferation in vitro.36,37 Also, vascular endothelial growth factor is stored within platelets, which indirectly stimulates hepatocyte proliferation by simulating HGF release from liver endothelial cells38 and likely directly supports liver regeneration by stimulating neoangiogenesis.39 It has, however, never been directly demonstrated that release of these growth factors occurs following platelet accumulation in the sinusoids. Similarly, evidence that platelet-derived growth factors are mandatory for platelet-mediated liver regeneration is still lacking.
It has been conclusively demonstrated that liver regeneration is delayed in mice deficient in circulating serotonin.10 These findings, however, are not necessarily compatible with a direct mitogenic effect of serotonin toward hepatocytes. An alternative explanation for the delayed regenerative response in mice lacking serotonin within their platelets is a defect in secondary platelet activation. Serotonin is a relevant platelet activator, and serotonin deficiency in platelets is associated with decreased functional platelet responses.40 Platelet activation is required for platelet-mediated liver regeneration, as P2Y12 inhibition also delays liver regeneration.10 Therefore, reduced platelet activation rather than defective mitogenic activity may explain the delayed liver regeneration in mice lacking platelet serotonin. A study in humans provided evidence for serotonin consumption following a partial hepatectomy, and showed an association between preoperative platelet serotonin content and outcome.41 This study, however, did not address whether serotonin consumption was directly related to the partial hepatectomy or simply the consequence of major (abdominal) surgery. Indeed, a study in which platelet serotonin content was compared between patients undergoing a partial hepatectomy and a pancreas resection provided evidence that a reduction of platelet serotonin following partial hepatectomy is not a unique phenomenon and the same process is also seen after pancreatic resection.42
Another study in humans provided data suggestive of release of vascular endothelial growth factor (VEGF) and thrombospondin-1 from platelets following partial hepatectomy.33 The balance between VEGF, which stimulates, and thrombospondin-1, which suppresses regenerative responses, has been shown to be related to outcome. Again, this study did not control for effects of general (abdominal and oncologic) surgery on the parameters assessed, and conclusions have been challenged.43
Alternative mechanisms
As platelets accumulate rapidly within liver sinusoids following a partial liver resection, release of proliferative molecules appears a plausible mechanism explaining platelet-mediated liver regeneration. We have recently demonstrated that transfer of RNA from platelet to hepatocyte substantially contributes to of platelet-mediated stimulation of hepatocyte proliferation.29 Whether functional RNA transfer contributes to liver regeneration in vivo needs to be established, as does the exact RNA species (which may be coding or regulatory RNAs) involved.
The possibility that platelets do not directly stimulate liver regeneration, but act as intermediates has not widely been considered in literature. However, another potential mechanism underlying platelet-mediated liver regeneration is facilitation of the inflammatory response. It has been well established that liver regeneration is associated with a localized or generalized inflammatory response. Liver regeneration is impaired in mice lacking inflammatory cells or production of proinflammatory cytokines such as tumor necrosis factor-α.44,45 Because platelets are well known to attract inflammatory cells,5 it is not unlikely that the role of platelet in liver regeneration is by facilitation the inflammatory response. Indeed, recent work has demonstrated that platelets are key in influx of neutrophils and repair following a sterile inflammation induced by thermal injury in a mouse model.46
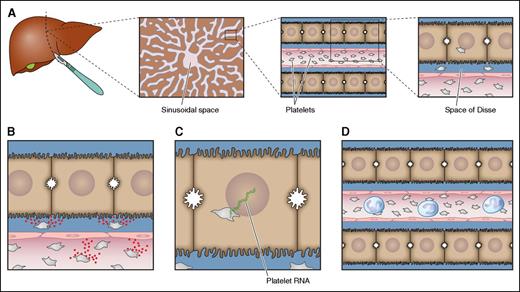
Potential mechanisms of platelet-mediated stimulation of liver regeneration are summarized in Figure 1.
Potential mechanisms underlying platelet-mediated liver regeneration. (A) Platelets accumulate in the sinusoidal space of injured livers or livers that have been surgically reduced in size. From the sinusoidal space, some platelets migrate into the Space of Disse and some platelets are taken up by hepatocytes. These platelets have the potential to activate multiple pathways that may all contribute to platelet-mediated stimulation of liver regeneration. Which of these pathways occur in vivo has not been definitively established. (B) Platelets may release contents from their granules that either directly stimulate hepatocyte proliferation (serotonin, IGF, and HGF) or stimulate endothelial cells to release HGF (VEGF). Alternatively, the direct interaction of platelets with endothelial cells promotes release of interleukin 6 and VEGF that promote liver regeneration. (C) Platelets may transfer their RNA to hepatocytes, which promotes hepatocyte proliferation either by translation of mRNA or by the action of regulatory RNAs. (D) Platelets attract inflammatory cells, which are known to directly stimulate liver regeneration. Professional illustration by Patrick Lane, ScEYEnce Studios.
Potential mechanisms underlying platelet-mediated liver regeneration. (A) Platelets accumulate in the sinusoidal space of injured livers or livers that have been surgically reduced in size. From the sinusoidal space, some platelets migrate into the Space of Disse and some platelets are taken up by hepatocytes. These platelets have the potential to activate multiple pathways that may all contribute to platelet-mediated stimulation of liver regeneration. Which of these pathways occur in vivo has not been definitively established. (B) Platelets may release contents from their granules that either directly stimulate hepatocyte proliferation (serotonin, IGF, and HGF) or stimulate endothelial cells to release HGF (VEGF). Alternatively, the direct interaction of platelets with endothelial cells promotes release of interleukin 6 and VEGF that promote liver regeneration. (C) Platelets may transfer their RNA to hepatocytes, which promotes hepatocyte proliferation either by translation of mRNA or by the action of regulatory RNAs. (D) Platelets attract inflammatory cells, which are known to directly stimulate liver regeneration. Professional illustration by Patrick Lane, ScEYEnce Studios.
Prospects
Adequate liver regeneration facilitates surgical procedures such as partial liver resections, living donor liver transplantation, and split liver transplantation. In addition, liver regeneration may result in spontaneous recovery from acute or chronic liver injury, and underlies recovery of full size liver grafts that have been damaged by cold and warm ischemia during the process of liver regeneration. Although regeneration proceeds adequately in many patients, in some patients the regenerative response is insufficient and results in liver failure which may necessitate emergency liver transplantation or result in death. In patients that underwent a partial hepatectomy or were transplanted with a partial liver graft, an incapacity of the liver to regenerate is referred to as the small-for-size syndrome.47,48 There is an unmet clinical need for therapeutic strategies to stimulate liver regeneration to treat or avoid the small-for-size-syndrome or to improve outcome of acute of chronic liver injury. The accumulating evidence on the importance of platelets in the regenerative response suggests that stimulating platelet-mediated liver regeneration may be of benefit for these patients.
Among potential therapeutic strategies are administration of platelet concentrates and thrombopoietin receptor agonists. Although experimental and limited clinical evidence suggests that these interventions may stimulate liver regeneration in humans, the risk/benefit ratio of such strategies may be unfavorable for three reasons. First, elevation of the platelet count may increase thrombotic risk. It has been demonstrated that platelet transfusions in patients with cancer increase the risk for both venous and arterial events,49 which may be related to a general increase in hemostatic potential by an increase in platelet count. In addition, the hypercoagulable state and imbalance between von Willebrand factor (VWF) and ADAMTS13 shortly after a partial hepatectomy has been suggested to contribute to venous and perhaps also portal vein thrombosis.50 This thrombotic risk may be exacerbated by elevated platelet counts, in particular in the context of the VWF/ADAMTS13 imbalance. Also, elevation of the platelet count in patients with cirrhosis has been shown to elevate the risk of portal vein thrombosis,51 which may also be linked to the VWF/ADAMTS13 imbalance of cirrhosis.52 Second, platelet transfusions may have undesirable side effects, notably transfusion-related acute lung injury. We have previously demonstrated an increase in mortality in liver transplant recipients who received perioperative platelet transfusions53 and demonstrated this increase in mortality to be attributable to an increased incidence of early postoperative acute lung injury.54 Third, although the stimulatory effects of platelets on liver regeneration have been well established, there may also be detrimental effects of platelets on liver function.19 For example, platelets have been shown to aggravate liver injury or fibrosis in mouse models of chronic and acute liver failure.55-58 In line with these results, a retrospective clinical study showed that use of aspirin was associated with a decreased progression of fibrosis in liver transplant recipients transplanted for hepatitis C (in whom fibrosis frequently recurs after transplantation).59 Furthermore, platelets contribute to ischemia/reperfusion injury,60 which is relevant in the context of liver transplantation and partial hepatectomy. Finally, platelets have been shown to promote cancer growth and metastasis,9 and therefore elevation of the platelet count may inadvertently promote cancer recurrence in patients that underwent a partial hepatectomy for (metastasized) primary or secondary liver malignancies and may promote cancer development in patients with cirrhosis.61
Thus, the (potential) risks of elevation of the platelet count do not justify clinical studies on prophylactic use of such strategies to stimulate liver regeneration in patients at risk for insufficient regenerative responses. Treatment of regenerative failure, for example, in patients with small-for-size-syndrome or patients with acute liver failure, may also not be justified as such patients frequently develop systemic inflammatory responses and multiorgan failure with associated hyperactivation of platelets.48,62,63 In these conditions, platelet transfusion may “fuel the fire.” Indeed, in patients with acute liver failure, those patients that receive platelet concentrate in the first week of their admission have a worse outcome than those that did not.64
Detailed studies on the mechanisms by which platelets stimulate liver regeneration will therefore be crucial in developing targeted therapeutic strategies to stimulate platelet-mediated liver regeneration.
Conclusion
Clinical and laboratory studies have provided convincing evidence of a stimulatory role of platelets in liver regeneration. However, the mechanisms underlying platelet-mediated stimulation of liver regeneration are incompletely understood.65 Mechanisms that have been postulated in literature are frequently only supported by circumstantial evidence. As platelets likely have both beneficial and detrimental effects in patients with liver injury, in patients that underwent surgical removal of part of the liver, or in patients that have been transplanted with a partial liver graft, therapeutic strategies should be targeted to promote beneficial effects while minimizing potential side effects.
Acknowledgments
Studies on platelet-mediated liver regeneration in our laboratory have been supported in part by a grant from The Netherlands Organisation for Scientific Research (VIDI, 917.11.304) (T.L.).
Authorship
Contribution: T.L. wrote the initial drafts of the manuscript; and R.J.P. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ton Lisman, Surgical Research Laboratory, Department of Surgery BA44, University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands; e-mail: j.a.lisman@umcg.nl.