To the editor:
Whether, and for which patients with acute myeloid leukemia, the dose of daunorubicin in induction should be 90 mg/m2 continues to be discussed. There seems little doubt that 90 mg/m2 is superior to a 45 mg/m2 dose.1-3 In this context, the reports from the Eastern Cooperative Oncology Group 1900 (E1900) trial suggest a benefit for patients <50 years of age who did not have adverse cytogenetics, and for patients who had mutations of DNMT3A, nucleophosmin 1 (NPM1), and mixed lineage leukemia-partial tandem duplication.1,4 A more recent follow up also showed a benefit for FLT3-internal tandem duplication (ITD) mutations.5 The concern however is that many investigators routinely use daunorubicin at a dose of 60 mg/m2, thus making extrapolations from this study about which patients need 90 mg/m2 as opposed to 60 mg/m2 less reliable.
We recently reported a large trial (n = 1206) comparing daunorubicin doses of 90 mg/m2 vs 60 mg/m2 in induction course 1.6 After course 1, patients defined as high risk, which was not based on FLT3 status, entered the high-risk arm of the trial, whereas the remaining patients received a second course that included 3 days of daunorubicin 50 mg/m2. The proportion of high-risk patients was the same in both the 60 mg/m2 and 90 mg/m2 arms. The trial independent Data Monitoring Committee recommended premature closure at a median follow-up of 14.8 months because of an excess risk of mortality by day 60 in the 90 mg/m2 arm, with no suggestion, on an intent to treat analysis, of later benefit. When reported, heterogeneity tests failed to identify any subgroup where there was significant interaction other than FLT3-ITD, but the effect of daunorubicin 90 mg/m2 + ara-C (DA90) failed to reach significance in this subgroup. We noted that this observation would require further follow up.
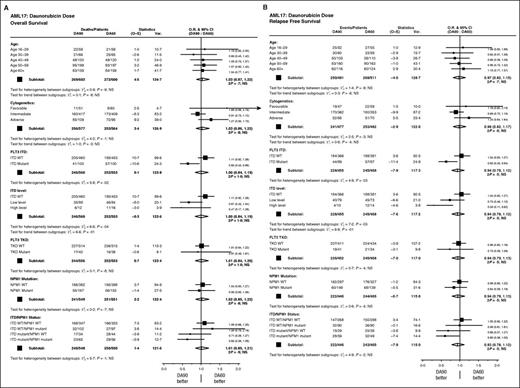
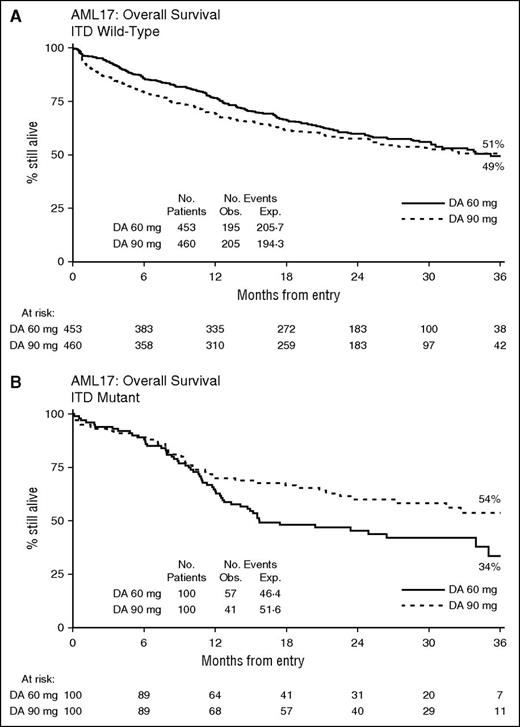
We have now re-analyzed the study, again by intention-to-treat, with median follow-up of 28 months. The outcomes (cumulative incidence of relapse [CIR], relapse-free survival [RFS], and overall survival [OS]) are virtually unchanged from the initial report with no differences emerging overall. Stratified analyses again show no significant interaction with treatment of any baseline feature, with the exception of patients with an FLT3-ITD mutation (Figure 1). Here, there was significant interaction on relapse, and RFS and OS with a significant benefit for 90 mg/m2 in FLT3-ITD mutant patients (CIR: 44% vs 60%; hazard ratio [HR], 0.58 (0.38-0.89); P = .01; RFS: 45% vs 33%; HR, 0.63 (0.43-0.94); P = .02; OS: 54% vs 34%; HR, 0.65 (0.43-0.96); P = .03; Figure 2). Although there is some evidence of increasing benefit on RFS with greater allelic burden, this is not seen when considering OS. The effect is unrelated to NPM1c mutations. Just over half (101/200) of the patients in this group were transplanted (50 vs 51); analyses of outcomes when censored at transplant are not significantly changed, although the point estimate is in favor of 90 mg/m2. The findings indicate that the benefit is due to a reduced risk of relapse in FLT3-ITD mutant patients treated at 90 mg/m2.
Stratified analyses of survival. (A) OS and (B) RFS. CI, confidence interval; NS, not significant; O–E, observed minus expected events; O.R., odds ratio; TKD, tyrosine kinase domain; Var, variance; WT, wild-type.
Stratified analyses of survival. (A) OS and (B) RFS. CI, confidence interval; NS, not significant; O–E, observed minus expected events; O.R., odds ratio; TKD, tyrosine kinase domain; Var, variance; WT, wild-type.
Survival by FLT3-ITD status. (A) ITD wild-type and (B) ITD mutant. Exp, expected events; Obs, observed events.
Survival by FLT3-ITD status. (A) ITD wild-type and (B) ITD mutant. Exp, expected events; Obs, observed events.
It is of course possible, as indicated in the detailed analysis of the E1900 trial, that there are further beneficiary patients who could be identified by more detailed molecular characterization, which has not been undertaken in our study.
Taken together, these data suggest that there is a place for escalated daunorubicin dosing for FLT3-ITD mutated cases. This presents a logistical challenge about the practicality of obtaining prompt FLT3-ITD mutation status without unduly delaying treatment. Our experience suggests that allelic ratio or NPM1 status is not needed to inform a treatment decision. Other options, which would require prospective evaluation, are to give the higher dose on days 3 and 5 of course 1, or even give the escalated dose in course 2 only. Developing therapies to improve the poor outcome for FLT3 mutated disease is an active area of clinical research, which has been given encouragement by the recent report by Stone et al of the RATIFY trial,7 which added midostaurin to conventional therapy and improved OS from 44.2% to 51.4% at 5 years, which is similar to the benefit that we show here (54% vs 34% overall at 3 years, and 53% vs 42% when censored at transplant) for the recipients of daunorubicin 90 mg/m2.
Authorship
Contribution: A.K.B. and R.K.H. designed the trial; A.K.B. wrote the paper; R.K.H. performed statistical analyses; A.K.B. and N.H.R. acted as chief investigators; and all authors contributed to data interpretation.
Conflict-of-interest disclosure: A.K.B. is employed by CTI Life Sciences. The remaining authors declare no competing financial interests.
A complete list of the members of the United Kingdom National Cancer Research Institute Acute Myeloid Leukemia Study Group appears in “Appendix.”
Correspondence: Alan K. Burnett, Department of Haematology, Cardiff University School of Medicine, Health Park, Cardiff CF14 4XN, United Kingdom; e-mail: akburnett719@gmail.com.
Appendix: study group members
The members of the United Kingdom National Cancer Research Institute Acute Myeloid Leukemia Study Group are: S. Ali, K. Bowles, D. Bowen, P. Cahalin, J. Cavenagh, R. Clark, C. Craddock, D. Culligan, M. Copland, M. Dennis, S. Freeman, R. Gale, B. Gibson, D. Grimwade, R. Hills, A. Hunter, B. Huntly, P. Johnson, G. Jones, H. Kaur, J. Kell, R. Kelly, A. Khwaja, L. Kjeldsen, S. Knapper, P. Kottaridis, M. F. McMullin, P. Mehta, M. Nikolousis, D. Richardson, N. Russell, D. Taussig, E. Tholouli, P. Vyas, and K. Wheatley.