Key Points
Treosulfan, a low-toxicity alkylating agent, can be used effectively as part of conditioning for HSCT in children with CGD.
Long-term follow-up is required to ascertain effects, particularly on gonadal function and compare with other regimens.
Abstract
Chronic granulomatous disease (CGD) can be cured by allogeneic hemopoietic stem cell transplantation (HSCT). Complications include graft failure, graft-versus-host disease (GVHD), infection, and transplant-related mortality; therefore, reduced-intensity conditioning regimens are being used to improve outcomes. In this retrospective study, the aim was to determine the outcome of treosulfan-based conditioning in HSCT for pediatric patients with CGD. The following data were collected: risk features pre-HSCT, additional conditioning agents, donor type and stem cell source, toxicity, engraftment, GVHD, chimerism, viral reactivation, post-HSCT complications, length of follow-up, and outcome. Seventy patients (median age, 107 months; interquartile range [IQR], 46-232 months) from 16 centers worldwide were transplanted between 2006 and 2015. Ninety-one percent had high-risk features. Fifty-seven HLA-matched donors, 12 HLA-mismatched donors, and 1 CD3+TCR αβ/CD19 depleted parental haploidentical transplants were performed. No major toxicity was reported. Median times to neutrophil and platelet engraftment were 17 (IQR, 15-35) and 16 (IQR, 13-50) days. At a median follow-up of 34 months (IQR, 13-102 months), the overall survival was 91.4%, and event-free survival was 81.4%. The cumulative incidence of acute grade III-IV GVHD was 12%. Nine patients developed chronic GVHD. When split cell chimerism was available, 95% or more myeloid donor chimerism was documented in 80% of surviving patients. Secondary graft failure occurred in 12% of patients. Treosulfan-containing conditioning regimens can be used safely in HSCT for children with CGD and high-risk clinical features, achieving excellent survival with high myeloid chimerism. Further studies are needed to compare with other regimens and evaluate the long-term outcome, particularly on fertility.
Introduction
Chronic granulomatous disease (CGD) is a primary immunodeficiency in which mutations in genes encoding 1 of the 5 subunits of the enzyme nicotinamide adenine dinucleotide phosphate oxidase lead to failure of microbicidal oxygen metabolite generation.1 This causes impaired microbial killing, which leads to severe life-threatening bacterial and fungal infections. In addition, impairment in the regulation and termination of pro-inflammatory cytokine-mediated signals cause granuloma formation and inflammation.1 Despite rigorous antibiotic and antifungal prophylaxis and treatment of inflammatory complications, ongoing medical problems are common in pediatric and adult patients, with a significant disease-related mortality.2-5
Hematopoietic stem cell transplantation (HSCT) can cure CGD with resolution of infections and inflammatory complications.5-7 In addition, growth and quality of life are improved in transplanted patients compared with those treated conservatively.4,8 Historically, high-risk patients with ongoing infectious or active inflammatory complications at HSCT had considerable transplant-related mortality up to 38%.9,10 Efforts to reduce the toxicity of the conditioning regimen were complicated by high rates of autologous reconstitution and graft-versus-host disease (GVHD).10,11
Recently, there has been increasing interest in the use of reduced-intensity conditioning regimens for patients with primary immunodeficiency,12 and specifically for those with CGD.13 These regimens cause minimal toxicity and achieve high rates of cure, even in patients with underlying infections and/or organ dysfunction.
Treosulfan, a bifunctional alkylating agent with myeloablative and immunosuppressive effects, has been increasingly used as 1 of the main conditioning agents for HSCT for children with malignant and nonmalignant disorders in some European and US centers.14-18 It has a low-toxicity profile, with the most commonly reported acute toxicities being skin, including nappy rash; diarrhea; mucositis; and hepatic toxicity; however, these are generally mild, and importantly, veno-occlusive disease (VOD) is very rare.19 Long-term effects are not well-documented because of the relatively recent introduction of the drug for conditioning for HSCT.
The purpose of this retrospective analysis was to determine the outcome of treosulfan-based conditioning for patients with CGD. We report a multicenter pediatric series of 70 patients with CGD who underwent HSCT, using treosulfan as the main agent for conditioning.
Patients, materials, and methods
Data collection
Centers identified through the Inborn Errors Working Party of the European Society for Blood and Marrow Transplantation that had performed HSCT for CGD, using a treosulfan-based conditioning regimen, were asked to participate in the retrospective study.
Data were submitted for 70 patients from 16 centers in 9 countries worldwide (United Kingdom, Germany, Belgium, Poland, Czech Republic, Italy, Israel, United States, and Australia) after a questionnaire distributed by the Inborn Errors Working Party of the European Society for Blood and Marrow Transplantation.
The following data were collected: risk features before HSCT, additional conditioning agents to treosulfan, donor type, stem cell source and number, toxicity (presence of skin toxicity, neurotoxicity, or VOD), platelet and neutrophil engraftment, occurrence of acute GVHD (aGVHD) after Glucksberg criteria and/or chronic GVHD20 (cGVHD), donor chimerism with lineage-specific chimerism CD3, CD19/CD20 and CD15/CD33 when available, viral reactivation (cytomegalovirus [CMV], Epstein-Barr virus, adenovirus, human herpesvirus 6), other HSCT complications, length of follow-up, and outcome.
Data submission and analysis were performed between February 2014 and October 2015. For the analysis of the parameters platelet and neutrophil engraftment, occurrence of GVHD, and donor chimerism, 1 patient was excluded, as he died on day +1. The 8 patients receiving a second procedure were included in the analysis of GVHD, as none of them developed either aGVHD or cGVHD after the first transplant.
All patients or their guardians gave written consent according to local center and European Society for Blood and Marrow Transplantation guidelines.
Patient characteristics
Sixty-six of the 70 patients were male. Two patients with long histories of recurrent infection were 19 years old (232 months) at the time of HSCT, the rest were younger than 18 years, with a median age of 9 years (107 months; interquartile range [IQR], 46-232 months). Fifty-six had X-linked CGD, and 11 were reported as having autosomal recessive (AR) disease: 4 cytochrome b-245, α polypeptide (CYBA); 4 neutrophil cytosolic factor 1; and 1 neutrophil cytosolic factor 2. For the other 2 patients, even after being extensively investigated, no mutation was found; however, based on the family history, females affected, and dihydrorhodamine pattern, X-linked inheritance was excluded. For 3 patients, this information was not available.
All except 6 patients had ongoing or previous radiologically and microbiologically proven infection or autoinflammation, defined as high-risk criteria pre-HSCT. Among the 64 (91%) patients who had these high-risk criteria, 34 patients had more than 1. The most frequently reported complication was infection in 52 patients, with previous microbiologically proven Aspergillus in 12, followed by colitis in 35, chronic lung disease in 15, and failure to thrive in 10 patients. Other significant symptoms reported in 10 patients were bladder inflammation, previous splenectomy, McLeod phenotype, pericardial effusion, thymus abscesses, allergic bronchopulmonary aspergillosis, recurrent hemophagocytic lymphohistiocytosis, and brain lesions with the syndrome of inappropriate antidiuretic hormone secretion. In addition, 5 patients had failed a previous transplant, 1 of whom has been previously reported.21
Transplantation
Fifty-six patients received a transplant from an unrelated donor (URD), 13 from a 10/10 HLA-matched related donor (MRD), (12 siblings [matched sibling donor] and 1 family [matched family donor]), and 1 received a CD3+ TCR α β+/CD19+ depleted haploidentical parental transplant (Table 1). Among the URD recipients, 12 received grafts that were less than 10/10 HLA matched (11 URD [9/10] and 1 cord blood [4/6)], and 44 received 10/10 HLA-matched grafts.
Patient characteristics
| Patient . | CGD type . | Sex . | Age at HSCT, mo . | High-risk clinical . | Donor (HLA match) . | Stem cell source . | Conditioning used . | CD34/kg body weight, ×106 . | Toxicity . | GHVD, grade . | Complications . | Latest chimerism, % donor . | Outcome/length of follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CYBB | M | 4,4 | Yes | URD 10/10 | PBSC | Treo Cy ATG | 21.99 | N | Skin II | CMV | 100 | Alive/102 |
| 2 | CYBB | M | 6 | Yes | MSD | BM | Treo Flu TT | 8.50 | N | N | No major complication | 74 | Alive/39 |
| 3 | CYBB | M | 7 | No | URD 10/10 | BM | Treo Flu A | 12.50 | Resolved limited skin rash | N | Pneumonitis | T, 83; B, 100; CD15, 100 | Alive/21 |
| 4 | CYBB | M | 7 | Yes | MSD 10/10 | BM | Treo Flu TT | 15 | N | Resolved limited cGVHD | EBV | T, 98; CD15, 96 | Alive/7 |
| 5 | CYBB | M | 9,6 | Yes | URD 9/10 | PBSC | Treo Cy ATG | 6.34 | N | Skin II, generalized extensive cGVHD | CMV EBV | 100 | Died/11 |
| 6 | CYBB | M | 11 | Yes | URD 10/10 | PBSC | Treo Flu A | 29.40 | Resolved limited skin rash | N | No major complication | T, 71; B, 24; CD15, 66 | Alive/32 |
| 7 | CYBB | M | 12 | No | URD 10/10 | BM | Treo Flu ATG | 6.89 | N | Skin II | No major complication | 100 | Alive/39 |
| 8 | CYBB | M | 14 | Yes | URD 10/10 | PBSC | Treo Cy A | 5/3.3 | N | N | Pneumonitis; adenovirus HHV6 | 100 | Top up 2 mo; alive/79 |
| 9 | CYBB | M | 17 | Yes | Cord 4/6 | Cord | Treo Flu ATG TBI | 1.11 | N | Gut II | No major complication | 100 | Alive/25 |
| 10 | CYBA | M | 18 | Yes | URD 10/10 | BM | Treo Flu A | 1.84 | N | Skin, liver II | Adenovirus | 31 | Alive/13 |
| 11 | CYBB | M | 18 | Yes | URD 10/10 | PBSC | Treo Flu A | 1.60 | N | Skin II | No major complication | T, 40; CD15, 14 | Alive/57 |
| 12 | CYBB | M | 18 | Yes | URD 10/10 | BM | Treo Flu ATG | 20.45 | N | Skin II | CMV EBV | T, 89; B, 86; CD15, 100 | Alive/6 |
| 13 | CYBB | M | 12.8 | Yes | URD 10/10 | PBSC | Treo Flu Cy ATG | 17.86 | N | Skin/liver/gut IV | CMV | 100 | Died/2 |
| 14 | CYBB | M | 33 | No | URD 10/10 | BM | Treo Flu TT | 3.53 | N | N | No major complication | 100 | Alive/32 |
| 15 | CYBB | M | 35 | Yes | URD 10/10 | BM | Treo Flu ATG | 8.83 | N | N | No major complication | T, 95; B, 99; CD15, 100 | Alive/5 |
| 16 | NCF2 | M | 37 | Yes | URD 10/10 | PBSC | Treo Flu A | 10.10 | N | Skin I | CMV, Guillain Barre | 100 | Retransplanted 18 mo; alive/54 |
| 17 | CYBA | M | 44 | Yes | α β TCR depleted father | PBSC | Treo Flu TT A | 34.50 | Resolved limited skin rash | Skin I | Adenovirus HHV6 CMV EBV | T, 84; B, 100; CD15, 100 | Alive/11 |
| 18 | CYBB | M | 45 | Yes | URD 10/10 | PBSC | Treo Flu A | 10.00 | Resolved limited skin rash | Skin I | No major complication | 100 | Alive/46 |
| 19 | CYBB | M | 46 | Yes | URD 10/10 | BM | Treo Flu ATG | 7.77 | N | Skin II | Adenovirus CMV | 100 | Alive/34 |
| 20 | CYBB | M | 46 | Yes | MSD | BM | Treo Cy | 10.80 | N | N | No major complication | 54 | Alive/47 |
| 21 | NCF1 | M | 46 | Yes | URD 10/10 | BM | Treo Flu A | 4.40 | N | Skin II | Pneumonitis | T, 93; B, 100; CD15, 100 | Alive/51 |
| 22 | CYBB | M | 47 | Yes | URD 9/10 | BM | Treo Flu A | 2.34 | Idiopathic epilepsy | N | No major complication | NA | DLI + retransplanted 3 m; alive/46 |
| 23 | CYBB | M | 49 | Yes | URD 10/10 | BM | Treo Flu A | 10.67 | N | N | No major complication | 39 | Alive/10 |
| 24 | AR | M | 55 | Yes | URD 10/10 | PBSC | Treo Flu A | 9.95 | N | N | No major complication | 100 | Alive/4 |
| 25 | CYBB | M | 55 | Yes | MSD | BM | Treo Flu TT | 15.00 | N | N | EBV | 100 | Alive/76 |
| 26 | CYBB | M | 59 | Yes | URD 10/10 | PBSC | Treo Flu A | 6.80 | N | Gut II | Adenovirus CMV | 100 | Alive/21 |
| 27 | CYBB | M | 60 | Yes | URD 10/10 | PBSC | Treo Flu A | 20.04 | N | Skin II | No major complication | 100 | Alive/39 |
| 28 | CYBB | M | 63 | Yes | URD 9/10 | BM | Treo Flu A | 6.10 | N | N | AIHA CMV | NA | Retransplanted 46 mo; alive/50 |
| 29 | CYBB | M | 64 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 3.75 | N | N | EBV | 100 | Alive/5 |
| 30 | NCF1 | F | 74 | Yes | MFD | BM | RI Treo Flu | 10.40 | N | cGVHD, resolved | CMV colitis and pneumonitis; agranulocytosis | 100 | Alive/75 |
| 31 | CYBB | M | 75 | No | MSD | BM | Treo Flu TT | 12.00 | N | N | No major complication | 100 | Alive/69 |
| 32 | CYBB | M | 76 | Yes | MFD | BM | Treo Flu | 3.09 | N | N | No major complication | T, 47; B, 31; CD15, 40 | Alive/34 |
| 33 | CYBB | M | 79 | Yes | URD 10/10 | BM | Treo Flu | 7.42 | N | Gut III | No major complication | 100 | Alive/56 |
| 34 | CYBB | M | 86 | Yes | URD 10/10 | PBSC | Treo Flu | 9.40 | N | Skin II | Disseminated Aspergillus | 100 | Alive/54 |
| 35 | CYBB | M | 88 | Yes | URD 10/10 | PBSC | Treo Flu A | 13.00 | N | Skin II | No major complication | T, 98; B, 100; CD15, 100 | Alive/35 |
| 36 | CYBB | M | 103 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 1.70 | N | Skin I | Recurrent bilateral otitis media | 100 | Alive/71 |
| 37 | CYBB | M | 106 | Yes | URD 10/10 | PBSC | Treo Flu A | 14.00 | Resolved limited skin rash | Skin I | CMV | 100 | Alive/39 |
| 38 | CYBA | M | 107 | No | URD 9/10 | BM | Treo Flu TT | 6.94 | N | IV | Adenovirus HHV6 CMV EBV | 100 | Alive/18 |
| 39 | CYBB | M | 110 | Yes | MSD | BM | Treo Flu | 2.60 | NA | NA -event- | Capillary leak; macrohematuria | NA | Died day +1 |
| 40 | CYBB | M | 113 | Yes | URD 9/10 | BM | Treo Flu A | 0.60 | N | N | Immune pancytopenia | 89 | Alive/54 |
| 41 | CYBB | M | 114 | No | URD 10/10 | PBSC | Treo Flu A | 10.50 | N | N | Pneumonitis | T, 89; B, 85; CD15, 87 | Alive/49 |
| 42 | CYBB | M | 118 | Yes | URD 10/10 | PBSC | Treo Flu A | 4.50 | N | N | No major complication | 100 | Alive/29 |
| 43 | CYBB | M | 119 | Yes | MSD | BM | Treo Flu | 7.50 | N | Skin/liver/gut IV; extensive cGVHD | Adenovirus | 100 | Died/10 |
| 44 | CYBB | M | 128 | Yes | URD 10/10 | PBSC | Treo Flu A | 16.00 | N | Skin/liver/gut III | Adenovirus | 100 | Top up 23 m; died/23 |
| 45 | CYBB | M | 129 | Yes | URD 10/10 | PBSC | Treo Flu A | 28.78 | N | cGVHD, resolved | No major complication | 100 | Alive/60 |
| 46 | ND | M | 132 | Yes | URD 10/10 | PBSC | Treo Flu A | 5.29 | N | N | CMV | 100 | Alive/46 |
| 47 | CYBB | M | 132 | Yes | URD 10/10 | PBSC | Treo Flu TT A | 10.00 | Resolved severe perineal rash | N | No major complication | NA | DLI + retransplanted 5 mo; alive/23 |
| 48 | CYBB | M | 133 | Yes | URD 9/10 | BM | Treo Flu A | 3.90 | Proximal myopathy | N | CMV | 100 | Alive/46 |
| 49 | CYBB | M | 139 | Yes | URD 10/10 | PBSC | Treo Flu A | 15.72 | N | N | No major complication | 100 | Alive/4 |
| 50 | CYBB | M | 141 | Yes | URD 10/10 | PBSC | Treo Flu A | 7.65 | N | N | AIHA | NA | Retransplanted 7 mo; alive/34 |
| 51 | CYBB | M | 144 | Yes | URD 10/10 | PBSC | Treo Flu A | 5.10 | N | Skin II, resolved limited cGVHD | CMV HHV6 | 100 | Alive/46 |
| 52 | CYBB | M | 145 | Yes | URD 9/10 | BM | Treo Flu TT ATG | 3.40 | N | N | no major complication | NA | DLI 7 mo; alive/30 |
| 53 | NCF1 | F | 147 | Yes | URD 10/10 | PBSC | Treo Flu A | 10.49 | N | Skin I | Bilateral maxillary sinusitis | 100 | Alive/11 |
| 54 | CYBB | M | 147 | Yes | MSD | BM | Treo Cy | 4.40 | N | N | No major complication | 35 | Alive/38 |
| 55 | CYBB | M | 163 | Yes | URD 10/10 | PBSC | Treo Flu A | 16.27 | N | N | EBV, red cell aplasia | CD3, 61; CD15, 100 | Alive/14 |
| 56 | NCF1 | F | 165 | Yes | URD 10/10 | BM | RI Treo Flu | 4.90 | N | Skin I | EBV, CMV enteritis | 100 | Alive/75 |
| 57 | ND | M | 173 | Yes | URD 10/10 | BM | Treo Flu ATG | 8.28 | N | Skin II | No major complication | T, 95; CD15, 100 | Alive/33 |
| 58 | CYBB | M | 176 | Yes | URD 9/10 | PBSC | Treo Flu A | 8.80 | N | cGVHD, resolved | EBV | 100 | Alive/68 |
| 59 | CYBB | M | 177 | Yes | MSD | BM | Treo Flu TT | 11.00 | N | N | No major complication | 100 | Alive/10 |
| 60 | CYBB | M | 180 | Yes | URD 9/10 | PBSC | Treo Flu A | 17.78 | N | N | CMV, EBV | 100 | Alive/64 |
| 61 | CYBB | M | 190 | Yes | URD 10/10 | PBSC | Treo Flu TT C | 13.07 | Resolved abdominal rash | N | No major complication | 100 | Alive/45 |
| 62 | AR | M | 191 | Yes | MSD | BM | Treo Flu ATG | 2.97 | N | Skin II, controlled cGVHD on treatment | Multifocal AVN | 100 | Alive/33 |
| 63 | CYBB | M | 198 | Yes | URD 10/10 | PBSC | Treo Flu A | 16.65 | Resolved generalized rash | Skin I | CMV | 100 | Alive/15 |
| 64 | ND | M | 198 | Yes | URD 9/10 | PBSC | Treo Flu A | 5.90 | N | N | TMA CMV | 100 | Alive/34 |
| 65 | CYBB | M | 200 | Yes | MSD | BM | Treo Flu A | 5.30 | N | N | CMV | T, 72; B, 82; CD15, 100 | Alive/14 |
| 66 | CYBB | M | 202 | Yes | URD 10/10 | PBSC | Treo Flu A | 13.39 | N | Skin II | CMV | 100 | Alive/12 |
| 67 | CYBB | M | 206 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 2.10 | N | N | CMV EBV PTLD | 100 | Alive/10 |
| 68 | CYBB | M | 208 | Yes | URD 9/10 | PBSC | Treo Flu A | 18.45 | Resolved generalized rash | Skin, gut III | Adenovirus, disseminated aspergillosis | NA | Died/4 |
| 69 | CYBA | F | 232 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 5.00 | N | Skin, gut III | EBV -Rituximab- Suspected pulmonary aspergillosis | 100 | Alive/28 |
| 70 | CYBB | M | 232 | Yes | URD 10/10 | BM | Treo Flu ATG | 3.37 | N | Skin II; ongoing cGVHD on treatment | No major complication | 100 | Alive/41 |
| Patient . | CGD type . | Sex . | Age at HSCT, mo . | High-risk clinical . | Donor (HLA match) . | Stem cell source . | Conditioning used . | CD34/kg body weight, ×106 . | Toxicity . | GHVD, grade . | Complications . | Latest chimerism, % donor . | Outcome/length of follow-up, mo . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CYBB | M | 4,4 | Yes | URD 10/10 | PBSC | Treo Cy ATG | 21.99 | N | Skin II | CMV | 100 | Alive/102 |
| 2 | CYBB | M | 6 | Yes | MSD | BM | Treo Flu TT | 8.50 | N | N | No major complication | 74 | Alive/39 |
| 3 | CYBB | M | 7 | No | URD 10/10 | BM | Treo Flu A | 12.50 | Resolved limited skin rash | N | Pneumonitis | T, 83; B, 100; CD15, 100 | Alive/21 |
| 4 | CYBB | M | 7 | Yes | MSD 10/10 | BM | Treo Flu TT | 15 | N | Resolved limited cGVHD | EBV | T, 98; CD15, 96 | Alive/7 |
| 5 | CYBB | M | 9,6 | Yes | URD 9/10 | PBSC | Treo Cy ATG | 6.34 | N | Skin II, generalized extensive cGVHD | CMV EBV | 100 | Died/11 |
| 6 | CYBB | M | 11 | Yes | URD 10/10 | PBSC | Treo Flu A | 29.40 | Resolved limited skin rash | N | No major complication | T, 71; B, 24; CD15, 66 | Alive/32 |
| 7 | CYBB | M | 12 | No | URD 10/10 | BM | Treo Flu ATG | 6.89 | N | Skin II | No major complication | 100 | Alive/39 |
| 8 | CYBB | M | 14 | Yes | URD 10/10 | PBSC | Treo Cy A | 5/3.3 | N | N | Pneumonitis; adenovirus HHV6 | 100 | Top up 2 mo; alive/79 |
| 9 | CYBB | M | 17 | Yes | Cord 4/6 | Cord | Treo Flu ATG TBI | 1.11 | N | Gut II | No major complication | 100 | Alive/25 |
| 10 | CYBA | M | 18 | Yes | URD 10/10 | BM | Treo Flu A | 1.84 | N | Skin, liver II | Adenovirus | 31 | Alive/13 |
| 11 | CYBB | M | 18 | Yes | URD 10/10 | PBSC | Treo Flu A | 1.60 | N | Skin II | No major complication | T, 40; CD15, 14 | Alive/57 |
| 12 | CYBB | M | 18 | Yes | URD 10/10 | BM | Treo Flu ATG | 20.45 | N | Skin II | CMV EBV | T, 89; B, 86; CD15, 100 | Alive/6 |
| 13 | CYBB | M | 12.8 | Yes | URD 10/10 | PBSC | Treo Flu Cy ATG | 17.86 | N | Skin/liver/gut IV | CMV | 100 | Died/2 |
| 14 | CYBB | M | 33 | No | URD 10/10 | BM | Treo Flu TT | 3.53 | N | N | No major complication | 100 | Alive/32 |
| 15 | CYBB | M | 35 | Yes | URD 10/10 | BM | Treo Flu ATG | 8.83 | N | N | No major complication | T, 95; B, 99; CD15, 100 | Alive/5 |
| 16 | NCF2 | M | 37 | Yes | URD 10/10 | PBSC | Treo Flu A | 10.10 | N | Skin I | CMV, Guillain Barre | 100 | Retransplanted 18 mo; alive/54 |
| 17 | CYBA | M | 44 | Yes | α β TCR depleted father | PBSC | Treo Flu TT A | 34.50 | Resolved limited skin rash | Skin I | Adenovirus HHV6 CMV EBV | T, 84; B, 100; CD15, 100 | Alive/11 |
| 18 | CYBB | M | 45 | Yes | URD 10/10 | PBSC | Treo Flu A | 10.00 | Resolved limited skin rash | Skin I | No major complication | 100 | Alive/46 |
| 19 | CYBB | M | 46 | Yes | URD 10/10 | BM | Treo Flu ATG | 7.77 | N | Skin II | Adenovirus CMV | 100 | Alive/34 |
| 20 | CYBB | M | 46 | Yes | MSD | BM | Treo Cy | 10.80 | N | N | No major complication | 54 | Alive/47 |
| 21 | NCF1 | M | 46 | Yes | URD 10/10 | BM | Treo Flu A | 4.40 | N | Skin II | Pneumonitis | T, 93; B, 100; CD15, 100 | Alive/51 |
| 22 | CYBB | M | 47 | Yes | URD 9/10 | BM | Treo Flu A | 2.34 | Idiopathic epilepsy | N | No major complication | NA | DLI + retransplanted 3 m; alive/46 |
| 23 | CYBB | M | 49 | Yes | URD 10/10 | BM | Treo Flu A | 10.67 | N | N | No major complication | 39 | Alive/10 |
| 24 | AR | M | 55 | Yes | URD 10/10 | PBSC | Treo Flu A | 9.95 | N | N | No major complication | 100 | Alive/4 |
| 25 | CYBB | M | 55 | Yes | MSD | BM | Treo Flu TT | 15.00 | N | N | EBV | 100 | Alive/76 |
| 26 | CYBB | M | 59 | Yes | URD 10/10 | PBSC | Treo Flu A | 6.80 | N | Gut II | Adenovirus CMV | 100 | Alive/21 |
| 27 | CYBB | M | 60 | Yes | URD 10/10 | PBSC | Treo Flu A | 20.04 | N | Skin II | No major complication | 100 | Alive/39 |
| 28 | CYBB | M | 63 | Yes | URD 9/10 | BM | Treo Flu A | 6.10 | N | N | AIHA CMV | NA | Retransplanted 46 mo; alive/50 |
| 29 | CYBB | M | 64 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 3.75 | N | N | EBV | 100 | Alive/5 |
| 30 | NCF1 | F | 74 | Yes | MFD | BM | RI Treo Flu | 10.40 | N | cGVHD, resolved | CMV colitis and pneumonitis; agranulocytosis | 100 | Alive/75 |
| 31 | CYBB | M | 75 | No | MSD | BM | Treo Flu TT | 12.00 | N | N | No major complication | 100 | Alive/69 |
| 32 | CYBB | M | 76 | Yes | MFD | BM | Treo Flu | 3.09 | N | N | No major complication | T, 47; B, 31; CD15, 40 | Alive/34 |
| 33 | CYBB | M | 79 | Yes | URD 10/10 | BM | Treo Flu | 7.42 | N | Gut III | No major complication | 100 | Alive/56 |
| 34 | CYBB | M | 86 | Yes | URD 10/10 | PBSC | Treo Flu | 9.40 | N | Skin II | Disseminated Aspergillus | 100 | Alive/54 |
| 35 | CYBB | M | 88 | Yes | URD 10/10 | PBSC | Treo Flu A | 13.00 | N | Skin II | No major complication | T, 98; B, 100; CD15, 100 | Alive/35 |
| 36 | CYBB | M | 103 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 1.70 | N | Skin I | Recurrent bilateral otitis media | 100 | Alive/71 |
| 37 | CYBB | M | 106 | Yes | URD 10/10 | PBSC | Treo Flu A | 14.00 | Resolved limited skin rash | Skin I | CMV | 100 | Alive/39 |
| 38 | CYBA | M | 107 | No | URD 9/10 | BM | Treo Flu TT | 6.94 | N | IV | Adenovirus HHV6 CMV EBV | 100 | Alive/18 |
| 39 | CYBB | M | 110 | Yes | MSD | BM | Treo Flu | 2.60 | NA | NA -event- | Capillary leak; macrohematuria | NA | Died day +1 |
| 40 | CYBB | M | 113 | Yes | URD 9/10 | BM | Treo Flu A | 0.60 | N | N | Immune pancytopenia | 89 | Alive/54 |
| 41 | CYBB | M | 114 | No | URD 10/10 | PBSC | Treo Flu A | 10.50 | N | N | Pneumonitis | T, 89; B, 85; CD15, 87 | Alive/49 |
| 42 | CYBB | M | 118 | Yes | URD 10/10 | PBSC | Treo Flu A | 4.50 | N | N | No major complication | 100 | Alive/29 |
| 43 | CYBB | M | 119 | Yes | MSD | BM | Treo Flu | 7.50 | N | Skin/liver/gut IV; extensive cGVHD | Adenovirus | 100 | Died/10 |
| 44 | CYBB | M | 128 | Yes | URD 10/10 | PBSC | Treo Flu A | 16.00 | N | Skin/liver/gut III | Adenovirus | 100 | Top up 23 m; died/23 |
| 45 | CYBB | M | 129 | Yes | URD 10/10 | PBSC | Treo Flu A | 28.78 | N | cGVHD, resolved | No major complication | 100 | Alive/60 |
| 46 | ND | M | 132 | Yes | URD 10/10 | PBSC | Treo Flu A | 5.29 | N | N | CMV | 100 | Alive/46 |
| 47 | CYBB | M | 132 | Yes | URD 10/10 | PBSC | Treo Flu TT A | 10.00 | Resolved severe perineal rash | N | No major complication | NA | DLI + retransplanted 5 mo; alive/23 |
| 48 | CYBB | M | 133 | Yes | URD 9/10 | BM | Treo Flu A | 3.90 | Proximal myopathy | N | CMV | 100 | Alive/46 |
| 49 | CYBB | M | 139 | Yes | URD 10/10 | PBSC | Treo Flu A | 15.72 | N | N | No major complication | 100 | Alive/4 |
| 50 | CYBB | M | 141 | Yes | URD 10/10 | PBSC | Treo Flu A | 7.65 | N | N | AIHA | NA | Retransplanted 7 mo; alive/34 |
| 51 | CYBB | M | 144 | Yes | URD 10/10 | PBSC | Treo Flu A | 5.10 | N | Skin II, resolved limited cGVHD | CMV HHV6 | 100 | Alive/46 |
| 52 | CYBB | M | 145 | Yes | URD 9/10 | BM | Treo Flu TT ATG | 3.40 | N | N | no major complication | NA | DLI 7 mo; alive/30 |
| 53 | NCF1 | F | 147 | Yes | URD 10/10 | PBSC | Treo Flu A | 10.49 | N | Skin I | Bilateral maxillary sinusitis | 100 | Alive/11 |
| 54 | CYBB | M | 147 | Yes | MSD | BM | Treo Cy | 4.40 | N | N | No major complication | 35 | Alive/38 |
| 55 | CYBB | M | 163 | Yes | URD 10/10 | PBSC | Treo Flu A | 16.27 | N | N | EBV, red cell aplasia | CD3, 61; CD15, 100 | Alive/14 |
| 56 | NCF1 | F | 165 | Yes | URD 10/10 | BM | RI Treo Flu | 4.90 | N | Skin I | EBV, CMV enteritis | 100 | Alive/75 |
| 57 | ND | M | 173 | Yes | URD 10/10 | BM | Treo Flu ATG | 8.28 | N | Skin II | No major complication | T, 95; CD15, 100 | Alive/33 |
| 58 | CYBB | M | 176 | Yes | URD 9/10 | PBSC | Treo Flu A | 8.80 | N | cGVHD, resolved | EBV | 100 | Alive/68 |
| 59 | CYBB | M | 177 | Yes | MSD | BM | Treo Flu TT | 11.00 | N | N | No major complication | 100 | Alive/10 |
| 60 | CYBB | M | 180 | Yes | URD 9/10 | PBSC | Treo Flu A | 17.78 | N | N | CMV, EBV | 100 | Alive/64 |
| 61 | CYBB | M | 190 | Yes | URD 10/10 | PBSC | Treo Flu TT C | 13.07 | Resolved abdominal rash | N | No major complication | 100 | Alive/45 |
| 62 | AR | M | 191 | Yes | MSD | BM | Treo Flu ATG | 2.97 | N | Skin II, controlled cGVHD on treatment | Multifocal AVN | 100 | Alive/33 |
| 63 | CYBB | M | 198 | Yes | URD 10/10 | PBSC | Treo Flu A | 16.65 | Resolved generalized rash | Skin I | CMV | 100 | Alive/15 |
| 64 | ND | M | 198 | Yes | URD 9/10 | PBSC | Treo Flu A | 5.90 | N | N | TMA CMV | 100 | Alive/34 |
| 65 | CYBB | M | 200 | Yes | MSD | BM | Treo Flu A | 5.30 | N | N | CMV | T, 72; B, 82; CD15, 100 | Alive/14 |
| 66 | CYBB | M | 202 | Yes | URD 10/10 | PBSC | Treo Flu A | 13.39 | N | Skin II | CMV | 100 | Alive/12 |
| 67 | CYBB | M | 206 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 2.10 | N | N | CMV EBV PTLD | 100 | Alive/10 |
| 68 | CYBB | M | 208 | Yes | URD 9/10 | PBSC | Treo Flu A | 18.45 | Resolved generalized rash | Skin, gut III | Adenovirus, disseminated aspergillosis | NA | Died/4 |
| 69 | CYBA | F | 232 | Yes | URD 10/10 | BM | Treo Flu TT ATG | 5.00 | N | Skin, gut III | EBV -Rituximab- Suspected pulmonary aspergillosis | 100 | Alive/28 |
| 70 | CYBB | M | 232 | Yes | URD 10/10 | BM | Treo Flu ATG | 3.37 | N | Skin II; ongoing cGVHD on treatment | No major complication | 100 | Alive/41 |
A, alemtuzumab; AIHA, autoimmune hemolytic anemia; AR, autosomal recessive; ATG, anti-thymocyte globulin; AVN, avascular necrosis; BM, bone marrow; CGD, chronic granulomatous disease; CMV, cytomegalovirus; Cy, cyclophosphamide; CYBA, cytochrome b-245, α polypeptide; CYBB, cytochrome b-245, β polypeptide; DLI, donor lymphocyte infusion; EBV, Epstein-Barr virus; F, female; Flu, fludarabine; GVHD, graft-versus-host disease (C, chronic); HHV6, human herpesvirus 6; HLA, human leukocyte antigen; HSCT, hematopoietic stem cell transplantation; M, male; MFD, matched family donor; MSD, matched sibling donor; N, none; NA, not applicable; NCF1, neutrophil cytosolic factor 1; ND, not determined; PBSC, peripheral blood stem cells; RI, radioimmunotherapy; TBI, total body irradiation; TCR, T cell receptor; TMA, thrombotic microangiopathy; Treo, treosulfan; TT, thiotepa; URD, unrelated donor.
Patients received bone marrow (n = 36), G-CSF mobilized peripheral blood stem cells (n = 33), or umbilical cord blood (n = 1) grafts. The median number of CD34+ hematopoietic stem cells administered was 8.50 × 106/kg (IQR, 4.5-34.5 × 106/kg).
The choice of conditioning regimen was institutionally dependent, with treosulfan as the primary myeloablative agent. There were 2 main groups: 46 (66%) patients received treosulfan, fludarabine ± serotherapy with either antithymocyte globulin (ATG) or alemtuzumab, and 24 patients received other regimens, with 15 patients receiving treosulfan, fludarabine, thiotepa ± ATG or alemtuzumab.
Standard total doses of treosulfan were 42 g/m2 or 36 g/m2, guided mainly by age and center preference. Fifty-nine patients older than 12 months received 42 g/m2, and 7 received 36 g/m2. All 4 patients younger than 12 months received 36 g/m2. The administration was in 3 doses from day −6 to day −4. No pharmacodynamic parameters of treosulfan were evaluated.
Fifty-seven patients received either ATG (n = 18) or alemtuzumab (n = 39) compared with 13 who did not receive any serotherapy.
With regard to the dose and timing of additional conditioning agents and serotherapy, it was variable, depending on center preference.
For GVHD prophylaxis, 62 patients received cyclosporin A, alone or with either mycophenolate mofetil in 45 (1 with additional methylprednisolone) or methotrexate in 13 patients. Eight patients received tacrolimus and methotrexate.
Statistical analysis
Overall survival (OS) and event-free survival (EFS) were described by Kaplan-Meier estimates. The Log-rank test was applied for the comparison between transplants from HLA-matched related and unrelated donors. Significance of results was determined using χ-squared test with Yates correction, using 2×2 contingency tables (GraphPad Prism 6; GraphPad Software, Inc., La Jolla, CA).
Results
Survival
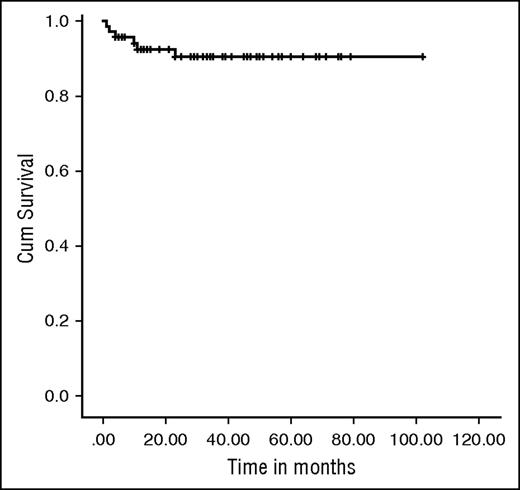
Sixty-four patients are alive, with a median follow-up of 34 (IQR, 13-102) months, giving an OS of 91.4% (Figure 1). The 2-year probability of survival was 90.48% (95% confidence interval, 79.86%-95.65%). There was no significant difference in OS between those who received an URD graft and those who received a MRD transplant (92.9% vs 85.7%; P = .255).
Kaplan-Meier survival curve. OS was 91.4% at a median follow-up of 34 months (IQR, 13-102 months).
Kaplan-Meier survival curve. OS was 91.4% at a median follow-up of 34 months (IQR, 13-102 months).
Of the 6 deaths resulting from transplant related mortality, only 2 occurred in the first 100 days post-HSCT, highlighting the low toxicity of the regimens (Table 2).
Deaths
| Age, mo . | Time posttransplant, mo . | High-risk features . | Donor . | Chimerism (%) . | Cause of death . |
|---|---|---|---|---|---|
| 110 | D+1 | Y | MSD | NA | Severe MOF Stroke |
| 114 | 11 | Y | URD 9/10 | 100 | Pneumonia cGVHD |
| 119 | 10 | Y | MSD | 100 | Adenovirus a/cGVHD G-IV |
| 128 | 23 | Y | MUD | 100 | Influenza pneumonitis aGVHD G-III |
| 152 | 2 | Y | MUD | 100 | Acute cardiac/pulmonary failure aGVHD G-IV |
| 208 | 4 | Y | URD 9/10 | 100 | Disseminated Aspergillus Disseminated Adenovirus |
| Age, mo . | Time posttransplant, mo . | High-risk features . | Donor . | Chimerism (%) . | Cause of death . |
|---|---|---|---|---|---|
| 110 | D+1 | Y | MSD | NA | Severe MOF Stroke |
| 114 | 11 | Y | URD 9/10 | 100 | Pneumonia cGVHD |
| 119 | 10 | Y | MSD | 100 | Adenovirus a/cGVHD G-IV |
| 128 | 23 | Y | MUD | 100 | Influenza pneumonitis aGVHD G-III |
| 152 | 2 | Y | MUD | 100 | Acute cardiac/pulmonary failure aGVHD G-IV |
| 208 | 4 | Y | URD 9/10 | 100 | Disseminated Aspergillus Disseminated Adenovirus |
GVHD, graft-versus-host-disease (a: acute; c: chronic); MOF, multiorgan failure; MSD, matched sibling donor; MUD, matched unrelated donor; URD, unrelated donor.
One patient with severe multisystem inflammatory disease and previous Aspergillus infection developed multiorgan failure during conditioning and died on day +1. The remaining 5 deaths were associated with severe GVHD, severe infection, or both (Table 2).
Toxicity
There were no serious toxicities with the exception of the expected chemotherapy-related myelosuppression. Nine patients had limited skin toxicity, including perianal ulceration, pigment changes, and occasional peeling. Two had central nervous system toxicity other than tremor related to cyclosporin A (1 idiopathic epilepsy, 1 proximal myopathy).
No VOD occurred, even in patients who had undergone previous HSCT.
Engraftment
Platelet engraftment (first day of platelet >20 × 109/L for 3 consecutive days) occurred at a median of 16 days (IQR, 13-50 days), and neutrophil engraftment (first day of neutrophils >0.5 × 109/L for 3 consecutive days) at 17 days (IQR, 15-35 days) post-HSCT. One patient died on day +1, and 1 patient who died at 10 months post-HSCT did not achieve platelet engraftment, despite 100% donor chimerism.
Viral reactivations
Thirty-four patients (48.5%) had viral reactivation, with 11 of them having more than 1 virus isolated in the blood. CMV was present in 22, Epstein-Barr virus in 14, adenovirus in 8, and human herpesvirus 6 in 4 patients. Disseminated adenovirus infection contributed to the death in 2 patients, and influenza pneumonitis in 1. Among these 34 patients, 29 had received previous serotherapy, either with alemtuzumab (n = 19) or ATG (n = 10).
Graft-versus-host disease
Twenty-seven patients (39%) developed aGVHD grade I-II, and the cumulative incidence of GVHD grade III-IV was 12% (8 patients). The incidence of grade III-IV GVHD was 7.6% for the MRD recipients (n = 1) and 12.5% for the URD recipients (n = 7). This difference was not statistically significant (P = .082).
Nine patients (13%) developed cGVHD: in 4 this was limited to the skin; in 3 it was extensive in the skin, joints, and muscle; and in 2 it was extensive in the skin, gut, and liver. In 5 patients, cGVHD has resolved and patients are off immunosuppression, 1 is receiving a weaning dose of immunosuppressive treatment with no symptoms, 1 has ongoing symptoms in spite of treatment, and 2 with extensive disease of skin, gut, and liver died. There was an unexpectedly higher cumulative incidence of cGVHD in the MRD group, at 30.7% (n = 4), compared with the URD group, at 16% (n = 9; P = .035). Three of these MRD recipients did not receive any serotherapy.
Among the URD recipients, there was no statistically significant difference in incidence of death, second procedures, severe aGVHD, or cGVHD between those recipients of mismatched unrelated donor and those who received matched unrelated donor grafts: there were 2 deaths in each group (P = .20) and 3 procedures in the mismatched unrelated donor compared with 5 in the matched unrelated donor recipients (P = .35). Two patients in the mismatched unrelated donor group developed aGVHD III-IV compared with 5 in the matched unrelated donor group (P = .64), and 2 in each group had cGVHD (P = .2).
There was a statistically significant difference in incidence of grade I-II aGVHD comparing serotherapy use versus none (ATG [n = 11] vs alemtuzumab [n = 16] vs nil [n = 0]; P = .002); this difference was not significant, depending on the serotherapy agent (ATG vs nil, P < .0001; alemtuzumab vs nil, P = .006; ATG vs alemtuzumab, P = .158).
The patients who received serotherapy and developed grade III-IV aGVHD or cGVHD were n = 5 and n = 6, respectively, compared with those who did not receive any; n = 3 for each type of GVHD, but the differences were not statistically significant (P = .19 for grade III-IV aGVHD and P = .305 for cGVHD).
Complications
Other significant complications reported were autoimmune hemolytic anemia (2 patients), immune pancytopenia (1 patient), thrombotic microangiopathy (1 patient), multifocal avascular necrosis (1 patient), Guillain-Barré syndrome (1 patient), and disseminated Aspergillus infection (1 patient).
Events and chimerism
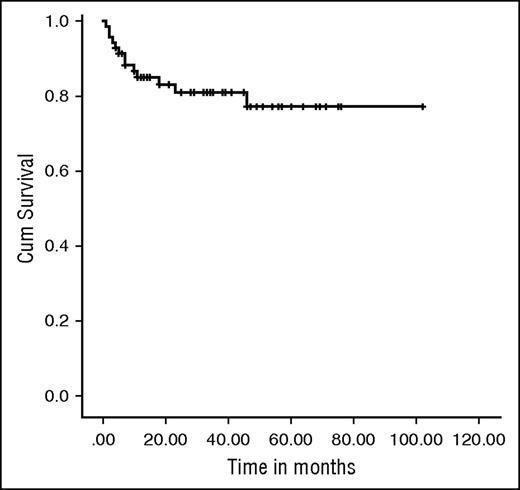
Eight patients required second procedures, resulting in an EFS of 81.4% at the median follow-up of 34 months (IQR, 13-102) (Figure 2). The 2-year probability of EFS was 81.03%, (95% confidence interval, 68.79%-88.85%). All these patients had received a total dose of treosulfan of 42 g/m2 with the exception of 1 who received 36 g/m2.
Kaplan-Meier EFS curve. EFS was 81.4% at a median follow-up of 34 months (IQR, 13-102 months).
Kaplan-Meier EFS curve. EFS was 81.4% at a median follow-up of 34 months (IQR, 13-102 months).
There was no significant difference between URD and MRD recipients (80.4% vs 85.7%; P = .490). Moreover, we found there was no difference in incidence of secondary graft failure with progressive decreasing chimerism in those patients with (4/22) or without (9/48) CMV reactivation (P = .9).
Second procedures included 2 boosts from the original donor without further conditioning; 1 of the patients died after developing severe extensive GVHD and disseminated adenovirus infection. Three received donor lymphocyte infusions, and 5 underwent a second HSCT (of whom 2 had received donor lymphocyte infusions) (Table 3).
Second procedures
| Age, mo . | Time of event posttransplant, mo . | High-risk features . | Conditioning . | CD34/kg body weight (×106) . | Stem cell source . | Donor . | Type of event . | Reason . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 14 | 2 | Y | Treo Cy A | 5.00 | PBSC | MUD | Top up | Decreasing donor chimerism | Alive, 100% chimerism |
| 37 | 18 | Y | Treo Flu A | 10.10 | PBSC | MUD | Second HCT: Bu/Flu | Decreasing donor chimerism | Alive, 100% chimerism |
| 47 | 3 | Y | Treo Flu A | 2.34 | BM | URD 9/10 | DLI ×3 + second HCT: 9/10 Cord, Bu/Flu | Decreasing donor chimerism (0%) | Alive, 100% chimerism |
| 63 | 46 | Y | Treo Flu A | 6.10 | BM | URD 9/10 | Second HCT: Bu/Flu | Decreasing donor chimerism | Alive. Stable donor chimerism |
| 128 | 23 | Y | Treo Flu A | 16.00 | PBSC | MUD | Top up | Pancytopenia with hypocellular marrow | Deceased |
| 132 | 5 | Y | Treo Flu TT A | 10.00 | PBSC | MUD | DLI ×3 + second HCT: Same donor Bu/Flu | Decreasing donor chimerism (20%) | Alive, 100% chimerism |
| 141 | 7 | Y | Treo Flu A | 7.65 | PBSC | MUD | Second HCT: Same donor Bu/Flu | Decreasing donor chimerism | Alive, stable donor chimerism |
| 145 | 7 | Y | Treo Flu TT ATG | 3.40 | BM | URD 9/10 | DLI ×5 | Decreasing donor chimerism | Alive, decreasing donor chimerism (CD15, 20%-40%; CD3, 70%-80%) |
| Age, mo . | Time of event posttransplant, mo . | High-risk features . | Conditioning . | CD34/kg body weight (×106) . | Stem cell source . | Donor . | Type of event . | Reason . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 14 | 2 | Y | Treo Cy A | 5.00 | PBSC | MUD | Top up | Decreasing donor chimerism | Alive, 100% chimerism |
| 37 | 18 | Y | Treo Flu A | 10.10 | PBSC | MUD | Second HCT: Bu/Flu | Decreasing donor chimerism | Alive, 100% chimerism |
| 47 | 3 | Y | Treo Flu A | 2.34 | BM | URD 9/10 | DLI ×3 + second HCT: 9/10 Cord, Bu/Flu | Decreasing donor chimerism (0%) | Alive, 100% chimerism |
| 63 | 46 | Y | Treo Flu A | 6.10 | BM | URD 9/10 | Second HCT: Bu/Flu | Decreasing donor chimerism | Alive. Stable donor chimerism |
| 128 | 23 | Y | Treo Flu A | 16.00 | PBSC | MUD | Top up | Pancytopenia with hypocellular marrow | Deceased |
| 132 | 5 | Y | Treo Flu TT A | 10.00 | PBSC | MUD | DLI ×3 + second HCT: Same donor Bu/Flu | Decreasing donor chimerism (20%) | Alive, 100% chimerism |
| 141 | 7 | Y | Treo Flu A | 7.65 | PBSC | MUD | Second HCT: Same donor Bu/Flu | Decreasing donor chimerism | Alive, stable donor chimerism |
| 145 | 7 | Y | Treo Flu TT ATG | 3.40 | BM | URD 9/10 | DLI ×5 | Decreasing donor chimerism | Alive, decreasing donor chimerism (CD15, 20%-40%; CD3, 70%-80%) |
A, alemtuzumab; BM, bone marrow; Bu, busulfan; Cy, cyclophosphamide; DLI, donor lymphocyte infusion; Flu, fludarabine; MUD, matched unrelated donor; PBSC, peripheral blood stem cells; Treo, treosulfan; TT, thiotepa; URD, unrelated donor transplant.
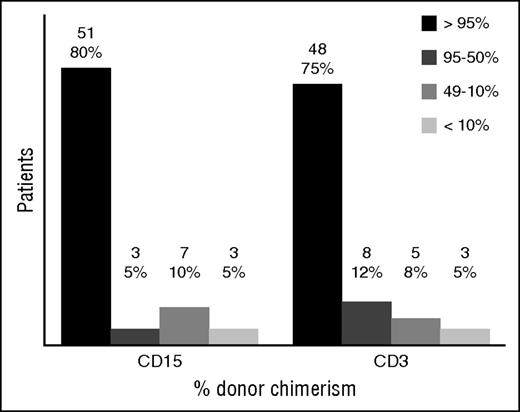
In 64 patients for whom data were available, last reported donor myeloid chimerism was higher than 95% in 51 patients (80%), and T lymphoid chimerism was higher than 95% in 48 patients (75%) (Figure 3). The remaining 6 patients were excluded for the following reasons: the patient who died on day +1; 1 of the patients who received an additional procedure for decreasing chimerism, whose chimerism pretransplant was not available; and 4 who had whole-blood chimerism below 100% but split chimerism was not reported. For these 4 patients, the whole-blood chimerism was 89%, 74%, 54%, and 39% respectively.
Split cell chimerism for CD15+ and CD3+ cells at last follow-up. Results given in absolute number and percentage over the 64 patients with available split chimerism. Those who had second procedures were included with their last result before the event. Those who died had the last result available before the death.
Split cell chimerism for CD15+ and CD3+ cells at last follow-up. Results given in absolute number and percentage over the 64 patients with available split chimerism. Those who had second procedures were included with their last result before the event. Those who died had the last result available before the death.
The patients did not have a neutrophil oxidative function performed routinely; myeloid chimerism higher than 50% can be considered enough for recovery of neutrophil function in the CGD.
Conditioning agents and serotherapy
All the centers used treosulfan as the main condition agent, but otherwise the regimens were quite heterogeneous; 15 patients had additional thiotepa, but there was no significant difference in terms of graft failure (n = 2; P = .794), higher percentage of myeloid chimerism (n = 12; P = .3146), or deaths (n = 0; P = . 3289) compared with those who did not receive thiotepa (n = 6, n = 34, and n = 6, respectively).
Fifty-seven patients received either ATG or alemtuzumab compared with 13 who did not receive any serotherapy.
No significant differences were found in deaths (n = 4 in the serotherapy group compared with n = 2 in nonserotherapy; P = .470) or graft failures (n = 8 vs n = 0, respectively; P = .140).
Genetic type
As stated earlier, there were 56 patients with X-linked CGD and 11 patients with autosomal recessive CGD. For 3 patients, the information was not available, so they have been excluded from this analysis. Of the X-linked patients with CGD, 51 had high-risk features pretransplant, as did 10 of the patients with AR disease. All deceased patients had X-linked CGD, as did 7 of the 8 patients who needed a second procedure. There is a 100% survival and 91% EFS in the AR group compared with 89.3% and 87.5%, respectively, in the X-linked group; these differences were not significant when analyzed (survival P = .255; EFS P = .75).
Discussion
Without HSCT, patients with CGD face life-threatening infections and other complications that lead to shortened life span and diminished life quality.2-5 Although HSCT is the only curative therapy, patients often have significant comorbidities, particularly molds and other infections that may contribute to transplant-related mortality. During the last 10 years, there have been multiple reports suggesting that treosulfan-based regimens were associated with reduced incidence of transplant-related mortality when used for conditioning patients with hematologic malignancies or other life-threatening disorders, including CGD.14,16-18 However, we report the largest series to date of children with CGD who underwent allogeneic HSCT using treosulfan-based conditioning.
With a median follow-up of 34 months, the OS was excellent, at 91.4%, with an EFS of 81.4% in a high-risk group of patients in this study (nearly all of the patients had significant pre-HSCT risk factors including infection or infections and/or inflammation). In addition, there were no differences in OS or EFS between matched and mismatched donor grafts or the addition of thiotepa to the conditioning regimen.
Because of the retrospective nature of the study, no toxicity scale was used, but in the reported cases, the toxicity was minimal; in particular, no VOD occurred even in patients receiving a second transplant. Two patients had neurotoxicity, and 9 had mild skin toxicity, which resolved.
Some comparisons can be made with the results shown by Güngör et al,13 who published the largest prospective study of a reduced-intensity conditioning regimen for HSCT for this disease in a cohort of pediatric and adult patients (median age, 12.7 years; IQR, 6.8-17.3 years). Using a sub-myeloablative total dose of busulfan, and at a median follow-up of 21 months, the OS was 93% and EFS 89%, with very promising results in terms of low toxicity, sustained chimerism, and therefore, recovery of neutrophil function.
Eight patients in our cohort experienced secondary graft failure, 1 of whom died. The remaining patients had stable donor chimerism in myeloid and lymphoid lineages at last follow-up, compatible with cure of the underlying disease. We did not find a relationship between graft failure and CMV reactivation, as was recently reported.22 In the study published by Güngör et al,13 3 patients experienced graft failure and received a second HSCT; 2 of them successfully and 1 who died at day 10 after HSCT. In this series, 5 patients had previous graft failure after conditioning with fludarabine and melphalan (n = 1) and busulfan (n = 4).
There were 6 deaths in our cohort, 2 in the first 100 days, and all but 1 were associated either with severe GVHD or severe viral infection, or both. We cannot draw any conclusions regarding differences in age, other conditioning agents used, or donor type.
Although the overall incidence of aGVHD was high, at 51%, the incidence of severe acute grade III-IV GVHD was low, at 8%, compared with 16% in a report of children with malignancy, using treosulfan-based conditioning.17 cGVHD developed in 9 patients (13%), 6 of whom are now free of symptoms. GVHD was not associated with use of serotherapy or the donor source. Further work needs to be done to determine optimal timing and dosing of serotherapy to minimize the risks for GVHD and viral reactivation.23
In Güngör's13 study, grade III-IV aGVHD was only 4%, and cGVHD was present in 4 of 56 patients (7%), 3 of which were of pediatric age. Viral reactivation was reported in almost half of the patients, but resolved with appropriate therapy, and contributed to death in 2 patients. This highlights the importance of monitoring and preemptive therapy for viral reactivation in patients receiving serotherapy.
These results show that HSCT using a treosulfan-based conditioning regimen is a safe treatment option in pediatric patients with CGD, even in those with high-risk clinical features pre-HSCT or those with no HLA-identical family donor, as has been previously recommended. We observed high curative rates and minimal toxicity with excellent survival, comparable to low-intensity conditioning regimens.24 A third of the patients in this study had additional agents to treosulfan, fludarabine, and serotherapy, and so further studies are needed to establish a consistent approach. The other main study using reduced-intensity conditioning published by Güngör et al13 is prospective and includes a number of adult patients (11 out of 56 were aged 19 years or older) and a smaller proportion of X-linked patients with CGD than our cohort (60% vs 83%). Our results suggest that patients with X-linked CGD have a somewhat higher mortality and less successful transplants than those with AR-CGD, although this did not reach statistical significance. This could contribute to the small differences in survival, transplant success, and GVHD between the 2 studies. Prospective studies will be required comparing treosulfan with low-dose busulfan-based regimens, but will need to have lengthy follow-up to determine any differences in chimerism and long-term toxicity. Further studies are also needed to evaluate the pharmacokinetics of treosulfan to determine any correlation with area under the curve and donor chimerism,19 and finally, studies are required to evaluate long-term toxicity, particularly looking at gonadal function.25
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Maddalena Migliavacca (Milan, Italy), Zoe Allwood (London, United Kingdom), and Graham Stewart (Glasgow, United Kingdom) for help providing patient data, and Intan Juliana Abd Hamid (Newcastle-Upon-Tyne, United Kingdom) for help with statistics.
The patients from the United States were enrolled in a clinical trial that was supported in part by grant HL122173 from the National Institutes of Health, National Heart, Lung, and Blood Institute.
Authorship
Contribution: B.M.-G., M.S., and A.R.G. designed the study, analyzed data, and wrote the paper; R.B., K.R., L.B., A.S., A.-M.E., B.G., P.S., L.K., B.S., I.Z., K.K., J.-A.T., A.W., C.F., I. Meyts, I. Müller, J.W., M.E.B., P.V., and K.-W.S. submitted data from their patients and reviewed and corrected the paper.
Conflict-of-interest disclosure: The patients from the United States were enrolled in a clinical trial that was supported in part by research funding from Medac, GmbH (Hamburg, Germany). In addition, Medac, GmbH provided Treosulfan for the study in the United States. J.W., K.-W.S., B.M.-G., A.R.G., and M.S. received travel grants from Medac.
Correspondence: Beatriz Morillo-Gutiérrez, Paediatric Infectious Diseases and Immunology Department, Great North Children's Hospital, Queen Victoria Rd, Newcastle upon Tyne NE1 4LP, United Kingdom; e-mail address: bmorillog@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal