Key Points
Peri-alloHCT IL-33 delivery prevents acute GVHD through MAPK-dependent expansion of radiation-resistant recipient ST2+ Tregs.
IL-33–expanded Tregs regulate myeloid cell differentiation and activation, and limit effector T-cell accumulation in GVHD-target tissue.
Abstract
During allogeneic hematopoietic cell transplantation (alloHCT), nonhematopoietic cell interleukin-33 (IL-33) is augmented and released by recipient conditioning to promote type 1 alloimmunity and lethal acute graft-versus-host disease (GVHD). Yet, IL-33 is highly pleiotropic and exhibits potent immunoregulatory properties in the absence of coincident proinflammatory stimuli. We tested whether peri-alloHCT IL-33 delivery can protect against development of GVHD by augmenting IL-33–associated regulatory mechanisms. IL-33 administration augmented the frequency of regulatory T cells (Tregs) expressing the IL-33 receptor, suppression of tumorigenicity-2 (ST2), which persist following total body irradiation. ST2 expression is not exclusive to Tregs and IL-33 expands innate immune cells with regulatory or reparative properties. However, selective depletion of recipient Foxp3+ cells concurrent with peri-alloHCT IL-33 administration accelerated acute GVHD lethality. IL-33–expanded Tregs protected recipients from GVHD by controlling macrophage activation and preventing accumulation of effector T cells in GVHD-target tissue. IL-33 stimulation of ST2 on Tregs activates p38 MAPK, which drives expansion of the ST2+ Treg subset. Associated mechanistic studies revealed that proliferating Tregs exhibit IL-33–independent upregulation of ST2 and the adoptive transfer of st2+ but not st2− Tregs mediated GVHD protection. In total, these data demonstrate the protective capacity of peri-alloHCT administration of IL-33 and IL-33–responsive Tregs in mouse models of acute GVHD. These findings provide strong support that the immunoregulatory relationship between IL-33 and Tregs can be harnessed therapeutically to prevent GVHD after alloHCT for treatment of malignancy or as a means for tolerance induction in solid organ transplantation.
Introduction
Interleukin-33 (IL-33) is an IL-1 cytokine with poorly understood pleiotropic immunologic functions.1,2 It is expressed in the nucleus of epithelial cells in the steady state and upregulated in other cell types during inflammation and stress.2-5 Depending upon the immunological context into which it is released, IL-33 can exacerbate both type 1 and type 2 immunity. In quiescent environments, IL-33 acts on cells expressing the IL-33 receptor suppression of tumorigenicity-2 (ST2), such as eosinophils,6 basophils,7,8 mast cells,9 type 2 innate lymphoid cells (ILC2),10,11 and CD4+ effector T cells,12 to stimulate robust secretion of the type 2 cytokines IL-5 and IL-13. IL-33–driven type 2 responses are protective against parasites,13 however, IL-33–supported type 2 inflammation is involved in pathogenic allergic and fibrotic diseases.11,14-21 Conversely, IL-12, a proinflammatory cytokine produced rapidly by myeloid antigen-presenting cells after exposure to pathogens, induces the expression of ST2 on CD8+, as well as natural killer (NK) and NKT cells.22-24 IL-12 signaling increases expression of T-bet, a transcription factor required for induction of ST2 on both CD4+ and CD8+ T cells.22,25 The induction of ST2 on these type 1 effectors enables IL-33 to increase their proliferation and augment their interferon γ (IFNγ) production, which supports viral clearance.4,25
Allogeneic hematopoietic cell transplantation (alloHCT) is a common therapeutic modality for the treatment of hematologic malignancies. Unfortunately, the development of acute graft-versus-host disease (GVHD) is a complication that impacts upwards of 50% of recipients.26 Acute GVHD results from type 1–dominated donor alloimmune responses injuring the skin, liver, and gastrointestinal tract.26,27 Although typically treatable with immunosuppressants, GVHD causes significant morbidity, and the 15% of patients with refractory disease have mortality rates approaching 90%.27 Conditioning therapy, involving total body irradiation (TBI) and/or chemotherapy, is required to eliminate recipient lymphocytes and allow for engraftment of donor stem cells.28 TBI, however, also causes barrier tissue damage and ultimately generates a proinflammatory environment in the gut that supports GVHD.29 Using IL-33–30 and ST2-deficient31 mice, we revealed that IL-33 is elevated in GVHD-target barrier tissues by conditioning with TBI or chemotherapy and augments detrimental ST2+ donor T-cell–mediated type 1 alloimmune responses that caused lethal acute GVHD.32 Developing the means to counter the proinflammatory properties of IL-33 should lessen the burden of GVHD.
Regulatory T cells (Tregs) suppress the alloimmune responses underlying GVHD and adoptive transfer of Tregs has shown therapeutic efficacy in early-stage clinical trials.33-36 In addition to its potent type 1– and type 2–promoting capacity, a growing body of research supports a role for IL-33 in Treg immunobiology.37-42 Our group and others have described a subset of Foxp3+ Tregs that express ST237-39,42 and expand in response to IL-33.37-39,43 IL-33–expanded Tregs play a role in protecting murine heart allografts from both acute39 and chronic43 rejection. ST2+ Tregs exhibit an activated phenotype compared with their ST2− counterparts, even in the steady state, and display a superior ability to suppress CD8+ T-cell IFNγ production.38 In nonlymphoid compartments, ST2+ Tregs are present at increased frequencies and would be well positioned to respond to IL-33 released from damaged tissue.37,42 Despite these interesting observations, there are no definitive studies examining the impact of both donor and host IL-33–responsive Tregs after alloHCT. Also, given the ability of IL-33 to profoundly expand Tregs,37-39 we investigated whether expansion of recipient IL-33–responsive Tregs by peri-alloHCT administration of IL-33 could overcome the deleterious effects of IL-33 post-TBI and alloHCT and found this to be the case.
Methods
Mice
C57BL/6 (B6; H-2b), BALB/c (H-2d), BALB/c Thy1.1, BALB/c CD45.1, B6-Tg(Foxp3-DTR/EGFP)23.2Spar/Mmjax (Foxp3-DTR), and B6 Foxp3-IRES-mRFP (FIR) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) or National Cancer Institute (NCI) Mouse Repository (Frederick, MD). BALB/c st2−/− mice were obtained from Dr Andrew N. J. McKenzie31 (MRC Laboratory of Molecular Biology, Cambridge, United Kingdom).
TBI and pre-TBI Treg depletion studies
B6 mice were treated with phosphate-buffered saline (PBS) or 1 μg of recombinant mouse IL-33 (BioLegend, San Diego, CA) by intraperitoneal injection every day for 10 days. On day 11, mice received lethal TBI (1100 cGy). Mice were euthanized on day 2 or day 4 post-TBI for analysis (nonirradiated control mice were used as the day 0 time point). For Treg depletion studies, Foxp3-DTR mice received 15 ng/g diphtheria toxin (DT; Sigma-Aldrich, St. Louis, MO) on day −11, and continued every other day thereafter through day −1 concurrently with IL-33 administration (day −10 through day −1).
AlloHCT and GVHD
Female recipient mice were exposed to lethal TBI (B6, 1100 cGy; BALB/c, 700 cGy) 1 day prior to alloHCT. On day 0, recipient mice were given 1 × 107 T-cell–depleted (TCD) allogeneic bone marrow (BM) cells alone or with 2 × 106 CD90.1-purified splenic allogeneic T cells by IV injection. For IL-33 peri-alloHCT studies, recipient mice were treated with PBS or 1 μg per mouse IL-33 from day −10 through day +4. For Treg depletion studies, Foxp3-DTR recipient mice received DT as in the previous section beginning on day −11, and continued every other day thereafter through day +3 concurrently with IL-33 administration (day −10 through day +4). For Treg adoptive transfer GVHD studies, 2 × 106 CD4+CD25+ spleen and lymph node cells from wild-type (WT; st2+/+) or st2−/− BALB/c mice were transferred with 1 × 107 BALB/c TCD-BM and 4 × 106 CD25-depleted CD3+ T cells, at a ratio of 1 Treg-to-2 T effectors. Mouse survival and clinical GVHD score were assessed as described.32,44,45
Other detailed methods
All other methods are described in detail in the supplemental Methods (available on the Blood Web site).
Results
Peri-alloHCT delivery of IL-33 to recipients protects against acute GVHD
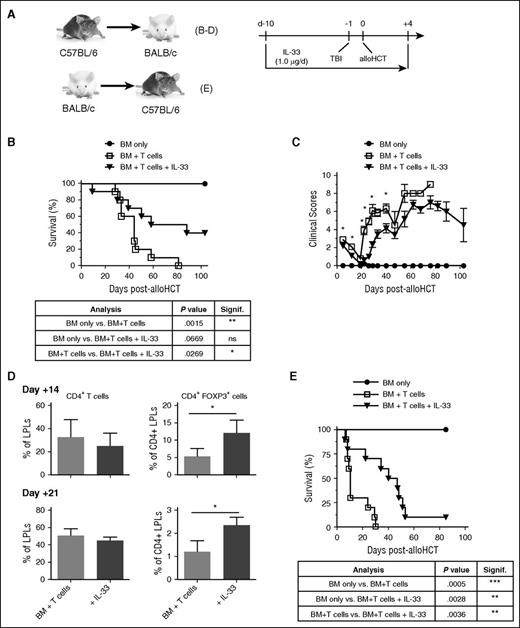
We have demonstrated that recipient IL-33 released post-TBI and alloHCT promotes acute GVHD through stimulation of type 1 alloimmune responses.32 The detrimental impact of released IL-33 was confirmed through alloHCT/GVHD experiments where administration of the IL-33 antagonist ST2-Fc, transplantation of st2−/− donor T cells, or transplant into il33−/− recipients, all prolonged survival and reduced type 1 alloimmune responses.32 Yet, administration of IL-33 prolongs cardiac allograft survival, potentially through expansion of Tregs39 or myeloid-derived suppressor cells (MDSCs),42 or induction of type 2 responses.43,46 Therefore, we tested whether peri-alloHCT conditioning of recipients with 1 μg of IL-33 (Figure 1A; day −10 through day +4) would protect against lethal acute GVHD. Compared with control mice, peri-alloHCT IL-33 administration prolonged BALB/c recipient mice survival (Figure 1B; mean survival time [MST] = 73 days for IL-33–treated mice vs MST = 44 days with vehicle alone; P = .0269). Approximately 50% of mice survived through day 100 post-alloHCT compared with vehicle-treated mice, which were all dead by day 80. Recipient protection from GVHD lethality was reflected in reduced clinical GVHD scores (Figure 1C). Assessment of GVHD-target tissues revealed that although the overall frequency of CD4+ T cells in the colon lamina propria (LP) lymphocytes (LPLs) was not altered, an increased frequency of CD4+Foxp3+ cells in the LPLs was evident on day 14 and day 21 (Figure 1D). The benefits of peri-alloHCT IL-33 were also verified in studies where donor and recipient strains were reversed (BALB/c to B6; Figure 1E; MST = 43.5 days for IL-33–treated mice vs MST = 10 days with vehicle alone; P = .0036).
IL-33 conditioning peri-alloHCT protects against acute GVHD. (A) Indicated combinations of WT mice were administered IL-33 (1.0 μg per mouse per day) starting on day −10 prior to alloHCT. On day −1, recipient mice received lethal TBI (B-D, 700 cGy to BALB/c recipients; E, 1100 cGy to B6 recipients) followed by 1 × 107 WT allogeneic TCD-BM (B-D, B6; E, BALB/c) alone or with 2 × 106 allogeneic pan T cells (B-D, B6; E, BALB/c) on day 0. IL-33 was continued through day +4 posttransplant. PBS vehicle-treated mice were used as BM + T only controls. (B,E) Survival curves with statistical significances calculated by log-rank (Mantel-Cox) test (*P < .05, **P < .01). For panels B-C, n = 4 (BM only), 10 (BM + T cells), and 10 (BM + T cells + IL-33) and the depicted experiment is representative of 2 independent experiments completed. For panel E, n = 5 (BM only), 10 (BM + T cells), and (10 BM + T cells + IL-33). (C) Group means with SEM are depicted for clinical GVHD scores. Significant differences for each point with ≥3 mice per group were calculated between BM + T cells vs BM + T cells + IL-33 using an unpaired Student t test (*P < .05). (D) Average frequency and standard deviation (SD) of indicated colon LPL population. Three to 4 mice per group with statistical significance calculated between groups using an unpaired Student t test (*P < .05). d, day; ns, not significant.
IL-33 conditioning peri-alloHCT protects against acute GVHD. (A) Indicated combinations of WT mice were administered IL-33 (1.0 μg per mouse per day) starting on day −10 prior to alloHCT. On day −1, recipient mice received lethal TBI (B-D, 700 cGy to BALB/c recipients; E, 1100 cGy to B6 recipients) followed by 1 × 107 WT allogeneic TCD-BM (B-D, B6; E, BALB/c) alone or with 2 × 106 allogeneic pan T cells (B-D, B6; E, BALB/c) on day 0. IL-33 was continued through day +4 posttransplant. PBS vehicle-treated mice were used as BM + T only controls. (B,E) Survival curves with statistical significances calculated by log-rank (Mantel-Cox) test (*P < .05, **P < .01). For panels B-C, n = 4 (BM only), 10 (BM + T cells), and 10 (BM + T cells + IL-33) and the depicted experiment is representative of 2 independent experiments completed. For panel E, n = 5 (BM only), 10 (BM + T cells), and (10 BM + T cells + IL-33). (C) Group means with SEM are depicted for clinical GVHD scores. Significant differences for each point with ≥3 mice per group were calculated between BM + T cells vs BM + T cells + IL-33 using an unpaired Student t test (*P < .05). (D) Average frequency and standard deviation (SD) of indicated colon LPL population. Three to 4 mice per group with statistical significance calculated between groups using an unpaired Student t test (*P < .05). d, day; ns, not significant.
IL-33 expands ST2+ Tregs and CD11b+F4/80+Gr-1int cells that persist following TBI
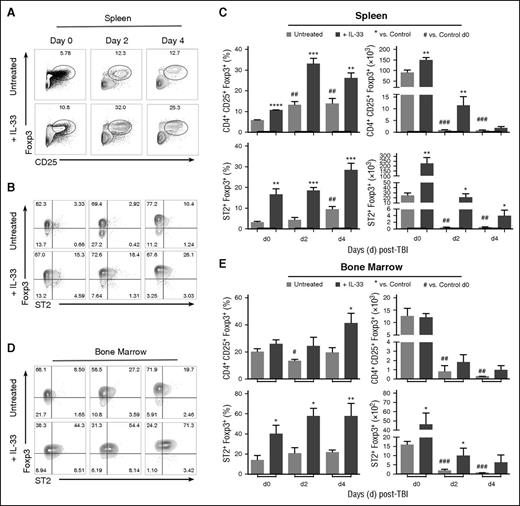
An ST2-expressing subset of Foxp3+ Tregs, which undergoes expansion following administration of IL-33, has been identified.38,39 Tregs also exhibit radiation resistance that elevates Foxp3+ cell frequency within the CD4+ T-cell population after exposure to TBI.47 When we exposed mice to a lethal dose of TBI, we recapitulated these data47 and established that the frequency of CD4+CD25hiFoxp3+ Tregs increased relative to total CD4+ T cells in the spleen through day +4 post-TBI (Figure 2A-C). The delivery of IL-33 for 10 days prior to TBI augmented this effect and an increased number of CD4+CD25+ Tregs was observed in the spleen (Figure 2C) at day 0 and day 2 post-TBI. These increases were not found in the BM (Figure 2E). As expected,38,39 IL-33 administration increased both the day 0 frequency and number of splenic ST2+ Treg cells (Figure 2B-C). Similar increases in ST2+ Tregs were observed in the BM (Figure 2D-E). ST2+ Tregs displayed considerable resistance to TBI, as the frequency of viable ST2+ Tregs remained increased in both locations (spleen; BM) in IL-33–treated mice through day +4 post-TBI (Figure 2). The frequency of ST2+ Tregs was also increased in untreated mice spleens at day +4 post-TBI (Figure 2B-C).
IL-33 and TBI expands ST2+ Tregs that persist through day 4 post-TBI. B6 mice were administered IL-33 (1.0 μg per mouse per day) or PBS (control) for 10 days and then exposed to lethal TBI (1100 cGy). Mice were sacrificed on day 2 or day 4 post-TBI, and nonirradiated mice were sacrificed on day 0. (A) Representative contour plots of CD3+CD4+-gated splenocytes showing Foxp3 vs CD25 expression from control (PBS) and IL-33–treated mice before and after TBI. (B) CD4+CD25+-gated splenocytes showing Foxp3 vs ST2 expression. (C) Summary graphs for the frequency (%) and cell number with SD the mean for the indicated populations. Average from n = 3-4 mice per group, and statistical significance was calculated between each group using an unpaired Student t test (*P < .05, **P < .01, ***P < .001); *IL-33–treated vs control at each time point; #significance for day 2 or day 4 control vs control day 0. Data are representative of at least 4 independent experiments. (D) CD4+CD25+-gated BM cells showing Foxp3 vs ST2 expression. (E) Summary graphs for the frequency (%) and cell number of indicated BM populations. Statistical significances were calculated as in panel C. Three to 4 mice per group and data are representative of 2 independent experiments.
IL-33 and TBI expands ST2+ Tregs that persist through day 4 post-TBI. B6 mice were administered IL-33 (1.0 μg per mouse per day) or PBS (control) for 10 days and then exposed to lethal TBI (1100 cGy). Mice were sacrificed on day 2 or day 4 post-TBI, and nonirradiated mice were sacrificed on day 0. (A) Representative contour plots of CD3+CD4+-gated splenocytes showing Foxp3 vs CD25 expression from control (PBS) and IL-33–treated mice before and after TBI. (B) CD4+CD25+-gated splenocytes showing Foxp3 vs ST2 expression. (C) Summary graphs for the frequency (%) and cell number with SD the mean for the indicated populations. Average from n = 3-4 mice per group, and statistical significance was calculated between each group using an unpaired Student t test (*P < .05, **P < .01, ***P < .001); *IL-33–treated vs control at each time point; #significance for day 2 or day 4 control vs control day 0. Data are representative of at least 4 independent experiments. (D) CD4+CD25+-gated BM cells showing Foxp3 vs ST2 expression. (E) Summary graphs for the frequency (%) and cell number of indicated BM populations. Statistical significances were calculated as in panel C. Three to 4 mice per group and data are representative of 2 independent experiments.
In addition to driving Treg expansion, IL-33 administration increases the frequency of myeloid cells, including MDSCs39,43 and macrophages.39 We confirmed IL-33 expansion of splenic CD11b+F4/80+Gr-1int cells (day 0; supplemental Figure 1A-B) that remain increased relative to control mice in number and frequency in the spleen through day 2 post-TBI (supplemental Figure 1A-B). Similar changes were not, however, observed in the BM (supplemental Figure 1C-D). The increase in splenic CD11b+F4/80+Gr-1int cells was associated with a corresponding decrease in frequency of CD11b+F4/80−Gr-1hi cells (supplemental Figure 1A-B). Expansion of the CD11b+F4/80+Gr-1int subset was dependent upon IL-33 administration, as TBI alone did not increase CD11b+F4/80+Gr-1int cells, but instead skewed the frequency of CD11b+ cells toward CD11b+F4/80−Gr-1hi cells (supplemental Figure 1A-B).
Tregs mediate the GVHD protection associated with peri-alloHCT delivery of IL-33
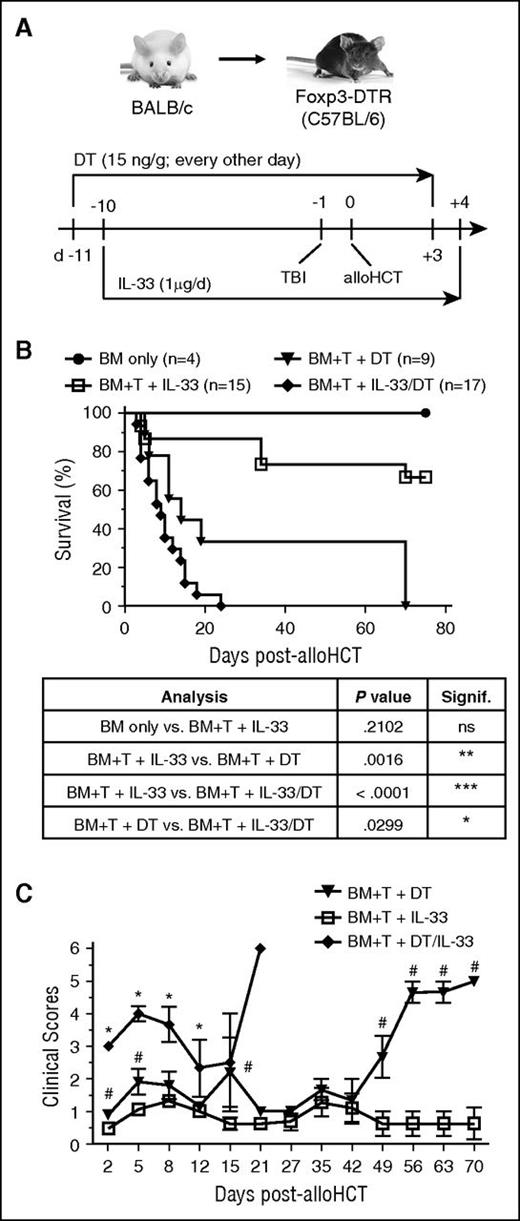
The capacity of IL-33 to prolong experimental cardiac allograft survival is dependent on Tregs, as antibody-mediated depletion of CD25+ cells before transplantation and IL-33 administration eliminates this therapeutic effect.39 To test the role of Tregs in protection against acute GVHD by peri-alloHCT delivery of IL-33, Foxp3-DTR recipient mice were administered DT concurrently during IL-33 administration (Figure 3A). Peri-alloHCT administration of IL-33 in Foxp3-DTR recipients resulted in ∼70% of mice surviving through day 80 posttransplant, whereas IL-33 delivery concurrent with depletion of Tregs significantly accelerated acute GVHD lethality as compared with DT alone (MST = 14 days vs 9 days, P < .03; Figure 3B). Accelerated GVHD lethality in IL-33 delivery with DT was reflected in an early elevation in clinical score (Figure 3C). These findings support the capacity of IL-33–expanded recipient Tregs in controlling early IL-33–driven inflammatory events that lead to acute GVHD.
Tregs mediate the GVHD-protective capacity of peri-alloHCT delivery of IL-33. (A) Foxp3-DTR B6 mice were administered 15 ng/g DT starting on day −11 (every other day) concurrently with daily IL-33 administration starting on day −10. On day −1, mice received lethal TBI (1100 cGy) followed by 1 × 107 WT BALB/c TCD-BM alone or with 2 × 106 BALB/c pan T cells on day 0. DT was continued through day +3 and IL-33 through day +4 posttransplant. (B) Resulting survival curves with statistical significances calculated by log-rank (Mantel-Cox) test (*P < .05, **P < .01, ***P < .001). (C) Recorded clinical scores are presented. Significant differences for each point with ≥3 mice were calculated between: (1) BM + T cells + IL-33 vs BM + T cells + DT/IL-33 (*P < .05) or (2) BM + T cells + DT vs BM + T cells + IL-33 (#P < .05) using an unpaired Student t test.
Tregs mediate the GVHD-protective capacity of peri-alloHCT delivery of IL-33. (A) Foxp3-DTR B6 mice were administered 15 ng/g DT starting on day −11 (every other day) concurrently with daily IL-33 administration starting on day −10. On day −1, mice received lethal TBI (1100 cGy) followed by 1 × 107 WT BALB/c TCD-BM alone or with 2 × 106 BALB/c pan T cells on day 0. DT was continued through day +3 and IL-33 through day +4 posttransplant. (B) Resulting survival curves with statistical significances calculated by log-rank (Mantel-Cox) test (*P < .05, **P < .01, ***P < .001). (C) Recorded clinical scores are presented. Significant differences for each point with ≥3 mice were calculated between: (1) BM + T cells + IL-33 vs BM + T cells + DT/IL-33 (*P < .05) or (2) BM + T cells + DT vs BM + T cells + IL-33 (#P < .05) using an unpaired Student t test.
Tregs restrain myeloid cell expansion and polarization following IL-33 administration
In addition to suppressors of other T cells, Tregs are potential regulators of myeloid cell–mediated inflammation. Treg depletion results in macrophage polarization toward the proinflammatory M1 phenotype after myocardial infarction.48 Likewise, Treg depletion causes accumulation of CD11bhiLy-6Chi inflammatory monocytes in injured muscle.49 Knowing that administration of IL-33 expands both splenic myeloid cells and Tregs and that Tregs are required for the therapeutic benefits of IL-33 against GVHD, we tested how Tregs influenced the myeloid compartment during IL-33 administration using Foxp3-DTR mice. Depletion of Tregs with DT or IL-33 administration alone increased splenocyte numbers compared with naive controls, and combined DT and IL-33 resulted in a threefold increase in total splenocytes (Figure 4A). Treg depletion by DT alone or with IL-33 was nearly complete (Figure 4B). As described in the preceding 2 paragraphs (supplemental Figure 1), IL-33 administration increased CD11bhi cells, predominantly the F4/80+Gr-1int subset (Figure 4D). Depletion of Tregs, both alone or with IL-33, greatly augmented the frequency of CD11b+F4/80−Gr-1hi cells (Figure 4D). Thus, IL-33 expanded potential monocytic MDSCs and macrophages (F4/80+Gr-1int cells), whereas Treg depletion instead promoted the expansion of neutrophils or granulocytic MDSCs (Gr-1hiF4/80−; Figure 4D). Confirming that these results were due to the elimination of Tregs and not as a result of DT toxicity, we verified that DT treatment of WT mice did not impact the myeloid or Treg compartments (supplemental Figure 2). When flow-sorted CD11b+F4/80+Gr-1lo and CD11b+F4/80−Gr-1hi cells were evaluated in a suppression assay, it was the Gr-1hi subset that possessed ex vivo T-cell–suppressive capacity (Figure 4E).
Tregs, including those expanded by IL-33, restrain the development of proinflammatory attributes by granulocytes and macrophages. Foxp3-DTR B6 mice were administered 15 ng/g DT on day −11 through day −1 (every other day) concurrently with IL-33 (day −10 through day −1). On day 0, spleens were harvested and splenocytes stained for multicolor flow cytometric analysis. (A) Assessed total splenocyte numbers for indicated groups (n = 3-4 mice per group): untreated (Untr.), DT only (DT), IL-33 only (IL-33), concurrent IL-33 and DT (DT/IL-33). (B) Representative contour plots and graphical analysis assessing Treg depletion during IL-33 treatment. Plots show Foxp3 vs CD25 expression on CD4+-gated cells. Graph presents the average frequency of CD25+Foxp3+ cells. (C) Representative flow plots and graphical analysis of CD11b+ cells in response to Treg depletion during IL-33 administration. (D) Representative flow plots and graphs showing F4/80 vs Gr-1 on CD11b+ cells. All graphs depict averages and SD from 3 to 4 mice per group and are representative of 2 independent experiments. Indicated significant differences were calculated using an unpaired Student t test (*P < .05, **P < .01, ***P < .01). (E) CD3−CD11b+F4/80+Gr-1lo and CD3−CD11b+ F4/80− Gr-1hi cells were flow sorted from the spleens of day 0 IL-33– or IL-33/DT-treated B6 Foxp3-DTR mice and assessed in an ex vivo suppression assay. Data represent the average and standard error of the mean (SEM) from 3 mice per group. Significant differences were calculated using an unpaired Student t test (*P < .05, **P < .01, ***P < .001, ****P < .0001). (F) Differential gene expression was also assessed by microarray between CD3−CD11b+F4/80+Gr-1lo cell populations from day 0 IL-33– or IL-33/DT-treated B6 Foxp3-DTR mice (n = 3 mouse per group). Partek calculated fold change values and associated P and q values were assessed using Ingenuity Pathway Analysis (IPA) and identified IFNγ as an active upstream regulator in CD3−CD11b+F4/80+Gr-1lo cells in the absence of Treg (z score, 2.137; P, 8.56E-06). The schematic is a graphical representation of the IFNγ signaling pathway in CD3−CD11b+F4/80+Gr-1lo cells from IL-33–/DT- vs IL-33 only-treated mice. The level of upregulation is indicated by intensity of red color at that node. Gray nodes are part of network, but were not significantly modified between IL-33– or IL-33/DT-treated B6 Foxp3-DTR mice samples. Solid lines indicate direct relationships; dashed lines depict indirect relationships. Yellow color represents predicted upstream regulators. CPM, counts per minute; GSK, glycogen synthase kinase; LTBR, lymphotoxin β receptor; MMP, matrix metalloproteinase; SSC, side scatter; TLR, Toll-like receptor; TNF, tumor necrosis factor.
Tregs, including those expanded by IL-33, restrain the development of proinflammatory attributes by granulocytes and macrophages. Foxp3-DTR B6 mice were administered 15 ng/g DT on day −11 through day −1 (every other day) concurrently with IL-33 (day −10 through day −1). On day 0, spleens were harvested and splenocytes stained for multicolor flow cytometric analysis. (A) Assessed total splenocyte numbers for indicated groups (n = 3-4 mice per group): untreated (Untr.), DT only (DT), IL-33 only (IL-33), concurrent IL-33 and DT (DT/IL-33). (B) Representative contour plots and graphical analysis assessing Treg depletion during IL-33 treatment. Plots show Foxp3 vs CD25 expression on CD4+-gated cells. Graph presents the average frequency of CD25+Foxp3+ cells. (C) Representative flow plots and graphical analysis of CD11b+ cells in response to Treg depletion during IL-33 administration. (D) Representative flow plots and graphs showing F4/80 vs Gr-1 on CD11b+ cells. All graphs depict averages and SD from 3 to 4 mice per group and are representative of 2 independent experiments. Indicated significant differences were calculated using an unpaired Student t test (*P < .05, **P < .01, ***P < .01). (E) CD3−CD11b+F4/80+Gr-1lo and CD3−CD11b+ F4/80− Gr-1hi cells were flow sorted from the spleens of day 0 IL-33– or IL-33/DT-treated B6 Foxp3-DTR mice and assessed in an ex vivo suppression assay. Data represent the average and standard error of the mean (SEM) from 3 mice per group. Significant differences were calculated using an unpaired Student t test (*P < .05, **P < .01, ***P < .001, ****P < .0001). (F) Differential gene expression was also assessed by microarray between CD3−CD11b+F4/80+Gr-1lo cell populations from day 0 IL-33– or IL-33/DT-treated B6 Foxp3-DTR mice (n = 3 mouse per group). Partek calculated fold change values and associated P and q values were assessed using Ingenuity Pathway Analysis (IPA) and identified IFNγ as an active upstream regulator in CD3−CD11b+F4/80+Gr-1lo cells in the absence of Treg (z score, 2.137; P, 8.56E-06). The schematic is a graphical representation of the IFNγ signaling pathway in CD3−CD11b+F4/80+Gr-1lo cells from IL-33–/DT- vs IL-33 only-treated mice. The level of upregulation is indicated by intensity of red color at that node. Gray nodes are part of network, but were not significantly modified between IL-33– or IL-33/DT-treated B6 Foxp3-DTR mice samples. Solid lines indicate direct relationships; dashed lines depict indirect relationships. Yellow color represents predicted upstream regulators. CPM, counts per minute; GSK, glycogen synthase kinase; LTBR, lymphotoxin β receptor; MMP, matrix metalloproteinase; SSC, side scatter; TLR, Toll-like receptor; TNF, tumor necrosis factor.
This suppressive capacity was reduced by Treg depletion, particularly with concurrent IL-33 delivery (Figure 4E). The CD11b+F4/80+Gr-1lo subset was not suppressive (Figure 4E). CD11b+F4/80+Gr-1lo cells from IL-33–treated and Treg-depleted mice were compared by microarray to IL-33–treated and Treg-sufficient mice. Cells from Treg-depleted mice displayed a transcriptional profile consistent with active IFNγ signaling and M1 macrophage polarization50 (increased STAT1, IRF1, IL-6, GBP3, -4, -7, and H2-Ab; Figure 4F). From these data, we concluded that Tregs, including IL-33–expanded Tregs, limit the frequency of CD11b+F4/80−Gr-1hi neutrophils and support granulocytic MDSC suppression. They do not, however, impact on the frequency of CD11b+F4/80+ macrophages, which are expanded by IL-33 and prone to M1 polarization in the absence of Tregs.
IL-33–expanded Tregs control myeloid cell activation and effector T-cell accumulation in GVHD-target tissue
Analysis of the myeloid cell compartment following IL-33 administration with Treg depletion showed that Tregs shape CD11b+ myeloid cell expansion and influence their differentiation. Further analysis on day 7 post-alloHCT revealed that, although IL-33 did not significantly alter the frequency of splenic CD11b+F4/80 or CD11b+Ly6Ghi cells over nontreated recipient mice (data not shown), IL-33 administration and associated Treg expansion supported a population of F4/80+CD11b+ cells with phenotypically reduced stimulatory capacity (Figure 5A). IL-33 delivery with Treg depletion instead resulted in F4/80+CD11b+ cells consistent with M1 macrophages50 as they displayed elevated surface expression of CD86 and major histocompatibility complex (MHC) class II (I-Ab) and intracellular IL-12p40 (Figure 5A). The extent of Treg expansion/deletion was confirmed in both the spleen and LP of the small intestine (SI), which is a key target tissue affected by GVHD (Figure 5B-C left panels). In the presence of Tregs, IL-33 treatment promoted accumulation of donor T cells in the spleen (Figure 5B right panel). In contrast, IL-33 administration with concurrent Treg depletion ablated donor T-cell splenic accumulation and permitted T-cell infiltration of the LP (Figure 5C right panel). These data indicate that IL-33 treatment expands Tregs that suppress macrophage maturation/M1 polarization, which may be important to reduce donor T-cell activation and exodus from the secondary lymphoid tissues. Likewise, IL-33–expanded Tregs protect against GVHD by suppressing effector T-cell infiltration of GVHD-target tissues.
IL-33–expanded recipient Tregs are critical regulators of macrophage activation and accumulation of effector T cells in GVHD-target tissue. Foxp3-DTR B6 mice were administered 15 ng/g DT starting on day −11 (every other day) concurrently with daily IL-33 administration starting on day −10. On day −1, mice received lethal TBI (1100 cGy) followed by 1 × 107 WT BALB/c TCD-BM alone or with 2 × 106 BALB/c pan T cells on day 0. DT was continued through day +3 and IL-33 through day +4 posttransplant. On day 7 post-alloHCT, splenocytes and LPLs isolated from the SI were subjected to flow cytometry. (A) Flow cytometric analysis of CD11b+F4/80+-gated splenocytes presented as mean with SD for MFI of CD86, MHC class II/I-Ab, and IL-12p40 on F4/80+CD11b+ cells (n = 5 per group). (B-C) Flow cytometric and graphical analysis of spleen (B) and SI LPLs (C) on day 7 post-alloHCT from mice treated with IL-33 alone or in combination with DT. (B) Left panel, CD4+Foxp3+-gated cells. Right panel, CD3+-gated cells. Analysis of Foxp3+ Tregs and CD4 and CD8 effector T cells. Statistical significance between groups was calculated by Student t test. In panels B-C, *P < .05, **P < .01 for DT/IL-33 vs IL-33. MFI, mean fluorescence intensity.
IL-33–expanded recipient Tregs are critical regulators of macrophage activation and accumulation of effector T cells in GVHD-target tissue. Foxp3-DTR B6 mice were administered 15 ng/g DT starting on day −11 (every other day) concurrently with daily IL-33 administration starting on day −10. On day −1, mice received lethal TBI (1100 cGy) followed by 1 × 107 WT BALB/c TCD-BM alone or with 2 × 106 BALB/c pan T cells on day 0. DT was continued through day +3 and IL-33 through day +4 posttransplant. On day 7 post-alloHCT, splenocytes and LPLs isolated from the SI were subjected to flow cytometry. (A) Flow cytometric analysis of CD11b+F4/80+-gated splenocytes presented as mean with SD for MFI of CD86, MHC class II/I-Ab, and IL-12p40 on F4/80+CD11b+ cells (n = 5 per group). (B-C) Flow cytometric and graphical analysis of spleen (B) and SI LPLs (C) on day 7 post-alloHCT from mice treated with IL-33 alone or in combination with DT. (B) Left panel, CD4+Foxp3+-gated cells. Right panel, CD3+-gated cells. Analysis of Foxp3+ Tregs and CD4 and CD8 effector T cells. Statistical significance between groups was calculated by Student t test. In panels B-C, *P < .05, **P < .01 for DT/IL-33 vs IL-33. MFI, mean fluorescence intensity.
IL-33–mediated ST2 signaling activates p38 MAPK to drive Treg expansion
Our investigations into the impact of IL-33 on Treg function revealed a selective expansion of ST2+Foxp3+ Tregs driven by IL-33–stimulated IL-2 production by CD11c+ dendritic cells (DCs).38 These findings did not probe whether IL-33 supported Treg induction or if its role was due to the expansion of thymic-derived Tregs. To address this point, we sorted CD4+CD25+Foxp3+ Tregs and CD4+Foxp3−CD62Lhi naive T cells (non-Tregs) from Foxp3-reporter mice. Culture with BALB/c CD11c+ BM-derived DCs (BMDCs) showed that both IL-2 and IL-33 exhibit a similar capacity to expand Tregs (supplemental Figure 4A). Although roughly a third of the proliferating cells (CTVlo) express ST2 in response to IL-2, ∼70% of the proliferating Tregs expressed ST2 in cultures with IL-33 (supplemental Figure 4A). In contrast, the addition of IL-33 to non-Treg cultures did not induce Foxp3 expression or support ST2 expression on these cells (supplemental Figure 4B). These data highlight the critical role of IL-33 in expansion, but not induction, of Tregs as assessed in vitro in this assay.
To establish the functional relationship between IL-33 and ST2 on Tregs after BM transplantation, we infused sorted ST2+ or ST2− Tregs into WT or il33−/− B6N mice 1 day after lethal irradiation. Recipient mice also received a syngeneic BM transplant to maintain IL-33+ or IL-33 knockout (KO) conditions. These studies revealed that, whereas ST2+ and ST2− Tregs display equal viability and both migrate to the spleen and BM (Figure 6A-C; supplemental Figure 5), ST2+ Tregs are more proliferative in both locations (Figure 6; supplemental Figure 5). More importantly, these studies establish that proliferating Tregs express higher levels of ST2, ST2− Tregs can induce ST2, and increased ST2 expression on Tregs does not require IL-33 (Figure 6A-B). Finally, these examinations demonstrated that recipient IL-33 drives the proliferation of ST2+ Tregs after transplantation (Figure 6; supplemental Figure 5).
IL-33 mediates p38 MAPK-dependent signaling to promote the expansion of proliferating Tregs expressing ST2. (A) Sorted ST2+ and ST2− CD4+ Foxp3(RFP)+Thy1.1+ cells from B6 OT-II FIR were labeled with CellTrace Violet (CTV), and infused into WT or il33−/− B6N mice that had been exposed to 1100 cGy 1 day prior. Recipient mice also received BM cells matched to recipient IL-33 status to create IL-33+ or IL-33 KO conditions. On day 5 posttransplant, splenocytes were isolated and CD90.1+ T cells assessed by flow cytometry for Foxp3 and ST2 expression, as well as proliferation (CTV dilution). Right flow plots, Proliferation and Foxp3 expression of the transferred CD90.1+ Tregs. Left panel, The ST2 expression on proliferating (P1) vs nonproliferating (P2) CD90.1+ cells. (B-C) Graphs present the average and SEM for (B) ST2 expression and (C) percentage proliferation (4 mice per group). Significant differences were calculated using unpaired Student t tests (*P < .05, **P < .01, ***P < .001, ****P < .0001). (D) CD4+Foxp3(RFP)+ Tregs were flow-sorted based on ST2 expression from B6 FIR mice and treated with IL-33 (100 ng/mL; bolded line) or (phorbol myristate acetate [PMA]/ionomycin; thin line) for 4 minutes before assessment for phospho-p38 or phospho-NF-κB p65 by flow cytometry. Representative histograms are presented. Unstimulated cells (filled histogram) served as a negative control. Graphs depict average calculated fold change in MFI of phospho-p38 or phospho-NF-κB p65 between IL-33–treated and untreated samples from 3 independent experiments. Statistical significance between groups calculated using the Student t test (*P < .05, **P < .01). (C) Bulk CD4+ T cells cultured with BALB/c CD11c+ BMDCs in media, stimulated with IL-33 alone, or IL-33 in combination with (E) the NF-κB inhibitors TPCA-1 or MG 132 or (F) the p38 MAPK inhibitor SB 203580. Flow plots depict ST2 expression vs CTV on CD3+CD4+Foxp3+-gated cells. (G) Average of results from 3 independent experiments represented in panel F. Statistical significance between groups calculated using the Student t test (*P < .05, **P < .01). AU, arbitrary unit.
IL-33 mediates p38 MAPK-dependent signaling to promote the expansion of proliferating Tregs expressing ST2. (A) Sorted ST2+ and ST2− CD4+ Foxp3(RFP)+Thy1.1+ cells from B6 OT-II FIR were labeled with CellTrace Violet (CTV), and infused into WT or il33−/− B6N mice that had been exposed to 1100 cGy 1 day prior. Recipient mice also received BM cells matched to recipient IL-33 status to create IL-33+ or IL-33 KO conditions. On day 5 posttransplant, splenocytes were isolated and CD90.1+ T cells assessed by flow cytometry for Foxp3 and ST2 expression, as well as proliferation (CTV dilution). Right flow plots, Proliferation and Foxp3 expression of the transferred CD90.1+ Tregs. Left panel, The ST2 expression on proliferating (P1) vs nonproliferating (P2) CD90.1+ cells. (B-C) Graphs present the average and SEM for (B) ST2 expression and (C) percentage proliferation (4 mice per group). Significant differences were calculated using unpaired Student t tests (*P < .05, **P < .01, ***P < .001, ****P < .0001). (D) CD4+Foxp3(RFP)+ Tregs were flow-sorted based on ST2 expression from B6 FIR mice and treated with IL-33 (100 ng/mL; bolded line) or (phorbol myristate acetate [PMA]/ionomycin; thin line) for 4 minutes before assessment for phospho-p38 or phospho-NF-κB p65 by flow cytometry. Representative histograms are presented. Unstimulated cells (filled histogram) served as a negative control. Graphs depict average calculated fold change in MFI of phospho-p38 or phospho-NF-κB p65 between IL-33–treated and untreated samples from 3 independent experiments. Statistical significance between groups calculated using the Student t test (*P < .05, **P < .01). (C) Bulk CD4+ T cells cultured with BALB/c CD11c+ BMDCs in media, stimulated with IL-33 alone, or IL-33 in combination with (E) the NF-κB inhibitors TPCA-1 or MG 132 or (F) the p38 MAPK inhibitor SB 203580. Flow plots depict ST2 expression vs CTV on CD3+CD4+Foxp3+-gated cells. (G) Average of results from 3 independent experiments represented in panel F. Statistical significance between groups calculated using the Student t test (*P < .05, **P < .01). AU, arbitrary unit.
We next sought to uncover the signaling pathways underlying ST2+ Treg IL-33–induced proliferation. In mast cells51,52 and basophils,7 IL-33 activates NF-κB and MAPKs (Erk1/2, p38, and JNK) downstream of ST2. We also observed phosphorylation of p38 MAPK and downstream NF-κB signals (p-p65) in flow-sorted ST2+ Tregs following IL-33 stimulation (Figure 6D). IL-33, however, did not cause phosphorylation of Erk1/2 or JNK (supplemental Figure 6). Although both are implicated in Treg proliferation,53,54 IL-33 did not modify STAT5 or mammalian target of rapamycin–related S6 kinase phosphorylation (supplemental Figure 6). The importance of p38 activity to ST2+ Treg proliferation was uncovered in subsequent studies utilizing a p38 MAPK-selective inhibitor and CD4+ T-cell culture with allogeneic BMDCs. Although IL-33 induced a nearly threefold increase in proliferating ST2+ Tregs stimulated with BMDCs compared with media alone, inhibition of p38 MAPK selectively blunted the proliferation of ST2+, but not ST2−, Tregs (Figure 6E-G). ST2 signaling resulted in NF-κB activation, however, NF-κB inhibition did not block ST2+ Treg expansion (Figure 6D-E). These data identify IL-33–mediated activation of p38 as a pivotal mechanism for ST2+ Treg expansion.
IL-33 responsiveness is critical for Treg-protective capacity against acute GVHD
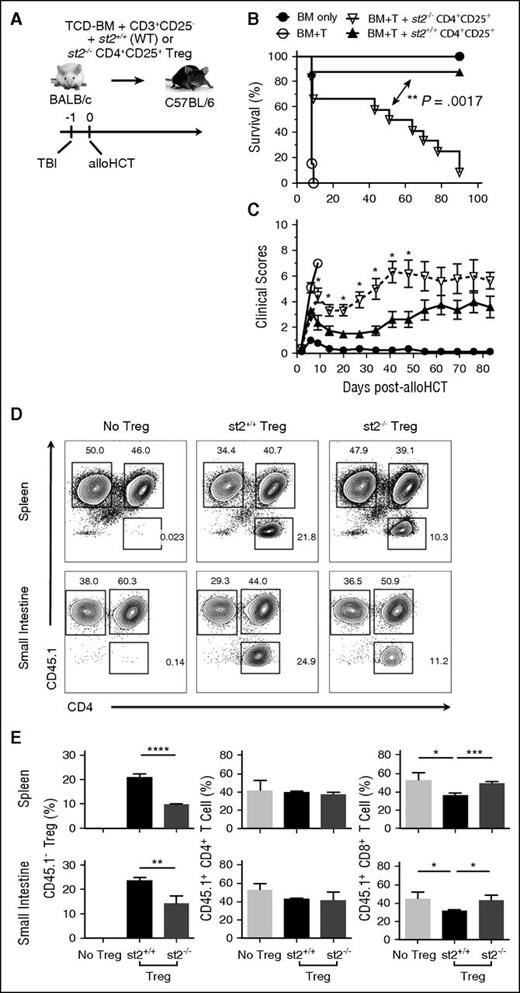
We demonstrated that endogenous IL-33 is upregulated and released from GVHD-target tissue post-TBI, where its dominant function was to drive lethal alloreactive donor T-cell responses.32 Although IL-33 expands Tregs38,39,41 and supports Treg stability,37 when physiologic ratios of donor Tregs to non-Tregs were transferred into alloHCT recipients, endogenous or exogenous IL-33 stimulation of Tregs posttransplant was insufficient to prevent lethal GVHD.32 The data depicted in Figures 1-3 suggest that, by increasing recipient Tregs before alloHCT with exogenous IL-33, we can tip the immunologic balance to where IL-33–responsive Tregs counter IL-33–driven alloreactive responses supporting GVHD. We wanted to test this concept further and directly assessed whether adoptively transferred Treg responsiveness to IL-33 contributes to their therapeutic capacity. Following TBI, purified st2+/+ (WT) or st2−/− CD4+CD25+ cells from BALB/c mice were adoptively transferred along with WT BALB/c donor TCD-BM and CD25-depleted T cells into WT B6 hosts (Figure 7). As expected,55,56 WT CD4+CD25+ cells protected against acute GVHD compared with mice receiving BM and effector T cells alone (Figure 7B; P < .001 vs BM + T). Conversely, st2−/− CD4+CD25+ cells displayed reduced protective capacity relative to WT Tregs (Figure 7B). Specifically, where WT Tregs promoted long-term survival in 90% of recipients compared, only 10% of mice that received st2−/− cells (Figure 7B; P = .0017, st2+/+ vs st2−/−) were protected. These findings are consistent with critical, IL-33–mediated protective Treg functions after alloHCT.
The IL-33–ST2 axis supports adoptively transferred Tregs and promotes their GVHD-protective capacity. (A) On day −1, WT B6 mice received lethal TBI (1100 cGy) followed by 1 × 107 WT BALB/c TCD-BM alone (n = 9) or with 4 × 106 BALB/c T cells (CD25-depleted) alone (BM+T; n = 13) or with 2 × 106 CD4+CD25+ Tregs from WT (st2+/+; n = 8) or st2−/− (n = 12) BALB/c mice on day 0. Effective Treg-to-T-effector ratio was 1:2. (B) Survival is depicted with significant differences calculated using the log-rank (Mantel-Cox) test. (C) Clinical scores were also monitored and statistical differences determined between st2−/− Tregs vs st2+/+ groups as in Figures 1 and 3. (D-E) B6 recipients irradiated as above received CD4+CD25+ T cells from st2+/+ or st2−/− BALB/c mice, as well as Thy1.1+ BALB/c TCD-BM, and CD25-depleted CD45.1+CD3+ T cells. (D) Representative flow plots depict CD45.1 and CD4 expression on Kd+Thy1.1−CD3+-gated splenocytes (top panels) or SI LPLs (bottom panels) at day 5 post-alloHCT. Full gating strategy is shown in supplemental Figure 7B. (E) Averages and SD are shown for the frequency of the indicated T-cell population in the spleen (top graphs) or SI LPLs (bottom graphs). Four mice per group and these data are representative of 2 independent experimental repeats. Statistical differences between groups were calculated using an unpaired Student t test (*P < .05, **P < .01, ***P < .001, ****P < .0001).
The IL-33–ST2 axis supports adoptively transferred Tregs and promotes their GVHD-protective capacity. (A) On day −1, WT B6 mice received lethal TBI (1100 cGy) followed by 1 × 107 WT BALB/c TCD-BM alone (n = 9) or with 4 × 106 BALB/c T cells (CD25-depleted) alone (BM+T; n = 13) or with 2 × 106 CD4+CD25+ Tregs from WT (st2+/+; n = 8) or st2−/− (n = 12) BALB/c mice on day 0. Effective Treg-to-T-effector ratio was 1:2. (B) Survival is depicted with significant differences calculated using the log-rank (Mantel-Cox) test. (C) Clinical scores were also monitored and statistical differences determined between st2−/− Tregs vs st2+/+ groups as in Figures 1 and 3. (D-E) B6 recipients irradiated as above received CD4+CD25+ T cells from st2+/+ or st2−/− BALB/c mice, as well as Thy1.1+ BALB/c TCD-BM, and CD25-depleted CD45.1+CD3+ T cells. (D) Representative flow plots depict CD45.1 and CD4 expression on Kd+Thy1.1−CD3+-gated splenocytes (top panels) or SI LPLs (bottom panels) at day 5 post-alloHCT. Full gating strategy is shown in supplemental Figure 7B. (E) Averages and SD are shown for the frequency of the indicated T-cell population in the spleen (top graphs) or SI LPLs (bottom graphs). Four mice per group and these data are representative of 2 independent experimental repeats. Statistical differences between groups were calculated using an unpaired Student t test (*P < .05, **P < .01, ***P < .001, ****P < .0001).
Our studies demonstrate that IL-33 drives ST2+ Treg proliferation ex vivo and in vivo. To characterize how a lack of IL-33–responsiveness impacts adoptively transferred Treg frequency, irradiated B6 recipients received CD4+CD25+ cells from st2+/+ or st2−/− BALB/c mice, Th1.1+ BALB/c TCD-BM, and CD25-depleted CD45.1+CD3+ T cells. Although overall spleen and SI LPLs numbers were unchanged (supplemental Figure 7), when splenocytes and LPLs from the recipients of st2+/+ CD4+CD25+ cells were assessed, increased frequency of donor Tregs was present in the spleen and SI compared with recipients of st2−/− CD4+CD25+ cells (Figure 7D-E). Consistent with our observation that IL-33–expanded Tregs protect against GVHD by suppressing effector CD8+ T-cell infiltration of GVHD-target tissues (Figure 5), mice receiving st2−/− Tregs also exhibited increased CD8+ T cells in the SI (Figure 7D-E). These findings suggest an important role for the IL-33/ST2 axis in increasing the frequency of adoptively transferred Tregs, which may support their protection against GVHD.
Discussion
Since it was first described as a type 2 cytokine inducer a decade ago,52 IL-33 has emerged as a pleiotropic IL-1 cytokine with the ability to augment type 14,22 responses and drive immune regulation.37-41 Although mechanisms controlling IL-33 pleiotropy are unclear, its activity is controlled by both the regulated availability of ST2-expressing cells, as well as the presence of proinflammatory cytokines.57,58 Recipient conditioning prior to alloHCT causes a breach in the intestinal epithelium that allows commensal bacteria to trigger proinflammatory cytokine secretion by local macrophages and DCs.27 Recipient-derived IL-33 released into this inflammatory milieu augments the type 1 donor alloimmune responses causing lethal acute GVHD.32 Data from solid organ transplant models, however, suggest that the regulatory properties of IL-33 can be harnessed to create a type 2/Treg-dominated environment that promotes allograft survival.39,43,46 Findings in other rodent models indicate that IL-33–stimulated Tregs are important for control of immune-mediated inflammation of the gut, but their immunoregulatory capacity may be limited by exposure to IL-23.37,59,60 These data fit with our current demonstration that recipient peri-alloHCT IL-33 treatment, well in advance of TBI-induced inflammation, provided Treg-mediated protection against acute GVHD. Likewise, in the absence of recipient Tregs, the effect of peri-alloHCT delivery mimicked that of post-alloHCT delivery,32 in that it accelerated GVHD lethality (Figure 3). Importantly, we also provide novel mechanistic observations establishing that the Treg-protective capacities against GVHD require IL-33 signaling and identify that IL-33 activation of p38 MAPK mediates expansion of the ST2+ subset of Tregs. In total, our current findings provide new appreciation for IL-33–responsive ST2+ Tregs in the prevention of GVHD and establish a potential pathway for the application of IL-33 immunotherapy in alloHCT.
In addition to a subset of lymphoid38,39 and tissue-resident Tregs,37,41 ILC2,61 as well as multiple myeloid cell subsets7 constitutively express ST2. Proinflammatory stimuli can also induce the expression of functional IL-33 receptor on cytotoxic CD8+ T cells4,38 and Th1 cells.25 In addition to ST2, the signaling transmembrane form, an IL-33-antagonistic soluble form (sST2), is generated by alternative splicing of messenger RNA.62-66 In the clinical setting, sST2 upregulated during GVHD is associated with increasing disease severity,67,68 and in mice, recent findings indicate that T cells, particularly the CD4+ Th17 subset, are a significant source of sST2 during GVHD.66 Our study, relying on depletion of Foxp3+ Tregs, is limited in that it cannot definitively establish that the protective role of peri-alloHCT IL-33 resides in the ST2+ subset of Tregs. However, we do reveal that among all the constitutive and potential ST2- and sST2-expressing cells of the body, Foxp3+ Tregs are crucial for the protective effect of peri-alloHCT IL-33.
Much focus has been placed on the importance of Tregs to limit effector T-cell proliferation and proinflammatory cytokine production in response to donor antigens.69,70 More recently, the ability of Foxp3+ Tregs to control the inflammatory influence of myeloid cells has gained recognition.48,49,71 In the current studies, macrophages were the primary population within the CD11b+ myeloid cell compartment expanded by IL-33 administration. This effect is supported by IL-33–expanded Tregs, which restrain the accumulation of neutrophils and support granulocytic MDSC function. Treg depletion concurrent with IL-33 delivery results in macrophage activation and M1 polarization, reflected in a CD11b+F4/80+Gr-1int cell gene expression profile consistent with IFNγ stimulation (Figure 4; supplemental Figure 3) and elevated macrophage expression of IL-12 and CD86 (Figure 5). Thus, our finding that a loss of Tregs results in increases in potentially detrimental M1-type macrophages parallel studies completed in models of sterile tissue damage.48,49 Likewise, IL-12hi and CD86hi macrophages could act as instigators of type 1 donor effector T-cell activation and mediate observed T-effector exit from the spleen and infiltration of GVDH-target tissues.
We first described an expansion of Tregs by IL-33 delivery that was required for IL-33 prolongation of cardiac allograft survival.39 Since then, reports of IL-33 contributing to Treg function and expansion in other models have grown considerably.37,41,49 We now demonstrate that IL-33–driven Treg expansion is maintained post-TBI, consistent with Treg radiation resistance.47 Effectively increasing the Treg-to-T-effector ratio is understood to be crucial for Treg control of alloimmune responses in the setting of transplantation and GVHD.70,72 Increasing Tregs to adequate protective numbers via adoptive transfer suppresses the alloimmune responses underlying GVHD and has shown therapeutic efficacy in preclinical rodent studies and early-stage clinical trials. For instance, adoptive transfer of ex vivo–expanded Tregs reduced grade II-IV GVHD.36,73 Low-dose IL-2 therapy is also being investigated as a therapeutic mechanism for in vivo Treg expansion to prevent acute GVHD following alloHCT.74 We now demonstrate that pretransplant expansion of recipient Tregs via IL-33 delivery mediates protection against acute GVHD that is associated with reduced M1 macrophage generation and decreased T-effector frequency. Although not presently investigated, ST2+ Tregs may also have IL-33–driven epithelial reparative capacities.40 Thus, carefully orchestrating expansion of ST2+ Tregs may be beneficial after alloHCT through both immune and nonimmune mechanisms. The findings of the present study, coupled with the recent work from our group32 and others,66,68 clearly establish the IL-33/ST2 axis, particularly as it relates to Treg immunobiology, as an attractive target for therapeutic intervention in the setting of alloHCT. We feel proper manipulation of the IL-33/ST2 axis after alloHCT will be critical to counter the IL-33–mediated detrimental type 1 alloimmune responses causing acute GVHD.32 Given the pleiotropic nature of IL-33 as discussed, and the recent implication of sST2 as an inflammatory mediator underlying GVHD,66 careful consideration must be given to appropriately shape donor and recipient immune responses to favor IL-33 immunoregulatory properties that can limit any detrimental IL-33 stimulation of the alloimmune responses causing GVHD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the Unified Flow Cytometry Core Facility at the University of Pittsburgh for their expertise and assistance. The authors thank Rosemary Hoffman for expertise and help with LPL isolation, as well as members of Angus W. Thomson’s laboratory for assistance with thymidine incorporation assays.
This work was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grants R00 HL097155 (H.R.T.), R01 HL122489 (H.R.T.), R01 HL56067 (B.R.B.), and T32 HL007062 (D.K.R.) and NIH National Institute of Allergy and Infectious Diseases grants R21 AI121981 (H.R.T.), R01 AI34495 (B.R.B.), R01 HL11879 (B.R.B.), and T32 AI074490 (B.M.M.). G.K.D. was supported by a grant from the NIH National Institute of General Medical Sciences (T32 GM008208). Further support was provided by the Heisenberg Professorship (DFG ZE 872/3-1) and DFG Einzelantrag (ZE 872/1-2) (R.Z.), an American Society of Transplantation/Pfizer Basic Science Faculty Development Grant (H.R.T.), and an American Society of Transplantation/Astellas Basic Science Postdoctoral Fellowship (B.M.M.).
Authorship
Contribution: B.M.M. designed and performed experiments, analyzed data, and wrote the manuscript; D.K.R., X.Z., G.K.D., L.M., J.M.L., B.H.K., F.M.U., and D.P. designed and performed experiments, analyzed data, and edited the manuscript; C.J.F., M.J.S., and Q.L. discussed experimental design and data analysis; R.Z. edited the manuscript; and B.R.B. and H.R.T. designed and analyzed experiments and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hēth R. Turnquist, Departments of Surgery and Immunology, Thomas E. Starzl Transplantation Institute, University of Pittsburgh School of Medicine, 200 Lothrop St, BST E1554, Pittsburgh, PA 15213; e-mail: het5@pitt.edu.
References
Author notes
B.M.M., D.K.R., and X.Z. are co-first authors.
B.R.B. and H.R.T. are co-senior authors.



![Figure 6. IL-33 mediates p38 MAPK-dependent signaling to promote the expansion of proliferating Tregs expressing ST2. (A) Sorted ST2+ and ST2− CD4+ Foxp3(RFP)+Thy1.1+ cells from B6 OT-II FIR were labeled with CellTrace Violet (CTV), and infused into WT or il33−/− B6N mice that had been exposed to 1100 cGy 1 day prior. Recipient mice also received BM cells matched to recipient IL-33 status to create IL-33+ or IL-33 KO conditions. On day 5 posttransplant, splenocytes were isolated and CD90.1+ T cells assessed by flow cytometry for Foxp3 and ST2 expression, as well as proliferation (CTV dilution). Right flow plots, Proliferation and Foxp3 expression of the transferred CD90.1+ Tregs. Left panel, The ST2 expression on proliferating (P1) vs nonproliferating (P2) CD90.1+ cells. (B-C) Graphs present the average and SEM for (B) ST2 expression and (C) percentage proliferation (4 mice per group). Significant differences were calculated using unpaired Student t tests (*P < .05, **P < .01, ***P < .001, ****P < .0001). (D) CD4+Foxp3(RFP)+ Tregs were flow-sorted based on ST2 expression from B6 FIR mice and treated with IL-33 (100 ng/mL; bolded line) or (phorbol myristate acetate [PMA]/ionomycin; thin line) for 4 minutes before assessment for phospho-p38 or phospho-NF-κB p65 by flow cytometry. Representative histograms are presented. Unstimulated cells (filled histogram) served as a negative control. Graphs depict average calculated fold change in MFI of phospho-p38 or phospho-NF-κB p65 between IL-33–treated and untreated samples from 3 independent experiments. Statistical significance between groups calculated using the Student t test (*P < .05, **P < .01). (C) Bulk CD4+ T cells cultured with BALB/c CD11c+ BMDCs in media, stimulated with IL-33 alone, or IL-33 in combination with (E) the NF-κB inhibitors TPCA-1 or MG 132 or (F) the p38 MAPK inhibitor SB 203580. Flow plots depict ST2 expression vs CTV on CD3+CD4+Foxp3+-gated cells. (G) Average of results from 3 independent experiments represented in panel F. Statistical significance between groups calculated using the Student t test (*P < .05, **P < .01). AU, arbitrary unit.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/3/10.1182_blood-2015-12-684142/4/m_427f6.jpeg?Expires=1765944745&Signature=Bqlg~--wLLArfYwqegHBnGbIZmzFWCvkOKI0nbBk-gu~Fira6DCltS4ombZQdYJDnX3Zd7cVHAJdygOhqUheH7Cqhbf6aSCSfM5X914p9Dmj9YfoUYuXiogHNTdnPktxhpMZojIBRolt9XVmSDIna5rtQI9USyEQu5Ig45WMz7R1kHWxLZCisPm~d17MnYeW0OqRt9RMWVuAJkB1xOMQlxbkIehqYmrbyX3WW1wGpWGjNA8HbO6L6LrfBbB3atu9viO8-TvsEHXTqVFxafvKpLp3-T5Rb38p59uUcXB0LtKNtlk2OeNjyNAM8bvahbuxY1S4p1ZeUaq~rKEdX9ag4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)