Key Points
Serum levels of VWF antigen are elevated in AL amyloidosis, reflecting endothelial dysfunction.
High VWF levels predict for poor outcome in patients with cardiac involvement and discriminate high-risk patients even within stage IIIB.
Abstract
Cardiac dysfunction determines prognosis in amyloid light-chain (AL) amyloidosis. The heart is the central organ of the vascular system in which endothelium function is critical for the circulatory homeostasis, but there are limited data on endothelial function in AL amyloidosis. von Willebrand factor (VWF) has been considered as a marker of endothelial activation and dysfunction, whereas a disintegrin and metalloproteinase with thrombospondin type-1 repeats 13 (ADAMTS-13) cleaves VWF multimers, but both have been associated with prognosis in cardiovascular disease. We measured the serum levels of VWF (VWF:Ag) and ADAMTS-13 antigens in 111 newly diagnosed patients with AL amyloidosis. The levels of VWF:Ag were significantly higher than in healthy controls; 76% of patients with AL had VWF:Ag levels higher than the upper levels of controls. There was no significant association of VWF:Ag levels with patterns of organ involvement, free light-chain levels, the levels of cardiac biomarkers, or renal dysfunction but correlated with low systolic blood pressure. VWF:Ag levels ≥230.0 U/dL were associated with higher probability of early death and poor survival independently of cardiac biomarkers and low systolic blood pressure (SBP). Moreover, among patients with Mayo stage III or stage IIIB (that is stage III with N-terminal pro-brain natriuretic peptide [NTproBNP] >8500 pg/mL) disease, VWF:Ag identified subgroups of patients with very poor outcome. Low ADAMTS-13 levels correlated with high levels of NTproBNP but had no independent prognostic significance. In conclusion, high VWF:Ag levels, probably representing endothelial dysfunction, are associated with prognosis in patients with AL amyloidosis, independently of other features of the disease or cardiac biomarkers.
Introduction
Organ dysfunction in systemic amyloid light-chain (AL) amyloidosis is caused by the deposition of amyloid fibrils in tissue microcirculation, disrupting tissue function and architecture.1 Recently, direct cellular toxicity of light chains or light-chain oligomers has been demonstrated in vitro and in vivo, and is most profound on cardiomyocytes or in nonvertebrate analog models.2-6 Cardiac dysfunction caused by AL amyloidosis is the main determinant of prognosis.7 However, myocardium is the central part of an extensive vascular system covered by functionally active endothelium, which is critical for the homeostasis of circulation.8 Endothelial cells (ECs) regulate multiple and complex functions of the vasculature, including vascular tone and coagulation in response to various stimuli, drugs, and toxins. Light chains may be directly toxic to the ECs and may thus alter vascular function.9-12
There are limited data on endothelial function in patients with AL amyloidosis.9,13 The study of the vascular function is complex, and surrogate markers of endothelial activation could be useful in understanding the potential role of endothelium in the development of symptoms and complications of AL amyloidosis. von Willebrand factor (VWF) is a large multimeric glycoprotein mainly produced (but not exclusively), stored, and secreted by ECs. VWF is critical for thrombus formation, but the secretion of VWF by ECs is triggered by several substances, and it has been proposed that changes in the levels of circulating VWF may reflect a state of “stimulation” of the endothelium.14-16 A disintegrin and metalloproteinase with thrombospondin type-1 repeats 13 (ADAMTS-13) cleaves the cell-bound large ultrapolymeric VWF strings. Acquired functional deficiency of ADAMTS-13 has been associated with thrombotic thrombocytopenic purpura,17 but a possible association of low levels of ADAMTS-13 antigen with arterial thrombosis and endothelial dysfunction has also been reported,18-23 but no data exist in patients with AL amyloidosis.
Thus, to evaluate the potential prognostic importance of VWF as a surrogate marker of endothelial “dysfunction,” we analyzed the outcomes of consecutive patients with AL amyloidosis in which the levels of VWF were measured before initiation of chemotherapy.
Patients and methods
Patients and controls
The analysis included 111 unselected consecutive patients with newly diagnosed, previously untreated AL amyloidosis. All patients were treated in a single center (Department of Clinical Therapeutics, Alexandra Hospital, Athens, Greece) receiving similar supportive care. All participants gave written informed consent for blood sampling and for recording of their medical data pertinent to the purposes of this study. Clinical and response data were collected prospectively in all patients, and all were assessed and followed rigorously according to a prespecified institutional protocol and received similar supportive care according to our institution’s practice. After venipuncture, serum was separated within 4 hours and stored at −80°C for all patients. Standard criteria were used for the definition of organ involvement and assessment of response.24,25 Serum samples from 40 otherwise healthy age-matched (±2 years) individuals were used as controls. Individuals with active infection or inflammatory conditions were excluded.
Measurements of cardiac biomarkers and VWF:Ag and ADAMTS-13:Ag in sera
NT-proBNP and hs-TnT measurements were performed based on the electrochemiluminescence immunoassay principle of Cobas e 411 analyzer (Roche Diagnostics, Mannheim, Germany). VWF antigen (VWF:Ag) levels were measured by means of a latex particle–enhanced immunoturbidimetric assay (HemosIL VWF antigen) with an automated coagulometer (ACL Top 3G, Instrumentation Laboratory, Lexington, MA). The inter- and intra-assay coefficients of variation were 2% and 3%, respectively, at a concentration of 123.5 U/dL, and the lower limit of detection was 2.2 U/dL. ADAMTS-13 antigen levels were measured by means of immunoenzymatic technique. According to the manufacturer inter- and intra-assay, coefficients of variation were <3.7 and <5.6, respectively, whereas the minimum detectable dose was 0.104 ng/mL (R&D Systems, Minneapolis, MN).
Statistical analysis
Differences among groups were compared with the χ2 test for categorical variables (using Fisher exact test when appropriate) and with the Mann-Whitney U test or analysis of variance for continuous variables. Time-to-event curves were plotted with the Kaplan-Meier method, and comparisons were made using the log-rank test. Overall survival was calculated from the date of initiation of therapy until the date of last follow-up or the date of death if it occurred earlier. Multivariate analysis was performed using logistic regression and Cox proportional hazards. SPSS 20 software was used for the statistical analysis.
Results
The characteristics of the patients that were included in the current analysis are depicted in Table 1. Their characteristics are typical of unselected amyloidosis patients: median age was 66 years (range 40-84) and the median number of involved organs was 2. The heart was involved in 65%, the kidneys in 71%, the peripheral nerve in 19%, the liver in 15%, and soft tissue in 21%. Median N-terminal pro-brain natriuretic peptide (NTproBNP) levels were 2297 pg/mL (range 33-75 000 pg/mL), and 20%, 49%, and 31% were Mayo stage I, II, and III, respectively; and 19% were Mayo stage III with NTproBNP levels ≥8500 pg/mL (stage IIIB). Primary therapy based on bortezomib was given in 59%, lenalidomide-based in 31%, and 10% received melphalan and dexamethasone. Median survival of the cohort was 34 months, and 3-, 6- and 12-month mortality was 15%, 24%, and 30%, respectively.
Characteristics of the patients in the analysis
| . | N = 111 patients . |
|---|---|
| Age, median (range) | 66 (40-84) |
| Males/females (%) | 60/40 |
| Organ involvement (%) | |
| Cardiac | 65 |
| Renal | 71 |
| Liver | 15 |
| PNS | 19 |
| Soft tissue | 21 |
| Number of involved organs, (range) | 2 (1-4) |
| NT-proBNP (pg/mL), median (range) | 2297 (17.0-121 000) |
| hsTnT (ng/L), median (range) | 42 (3-338) |
| Involved FLC (mg/L), median (range) | 235 (9-900) |
| Mayo stage (%) | |
| I | 20 |
| II | 49 |
| III | 31 |
| Stage IIIB (Mayo stage III and NTproBNP >8500 pg/mL) (%) | 19 |
| Proteinuria g/day, median (range) | 4.8 (0.5-39) |
| eGFR mL/min/1.73 m2, median (range) | 71 (8 to >150) |
| VWF U/dL, median (range) | 203.0 (20.0-1046.0) |
| IQR (range) | 127.0-235.0 |
| ADAMTS-13 ng/mL, median (range) | 1044 (770-1600) |
| . | N = 111 patients . |
|---|---|
| Age, median (range) | 66 (40-84) |
| Males/females (%) | 60/40 |
| Organ involvement (%) | |
| Cardiac | 65 |
| Renal | 71 |
| Liver | 15 |
| PNS | 19 |
| Soft tissue | 21 |
| Number of involved organs, (range) | 2 (1-4) |
| NT-proBNP (pg/mL), median (range) | 2297 (17.0-121 000) |
| hsTnT (ng/L), median (range) | 42 (3-338) |
| Involved FLC (mg/L), median (range) | 235 (9-900) |
| Mayo stage (%) | |
| I | 20 |
| II | 49 |
| III | 31 |
| Stage IIIB (Mayo stage III and NTproBNP >8500 pg/mL) (%) | 19 |
| Proteinuria g/day, median (range) | 4.8 (0.5-39) |
| eGFR mL/min/1.73 m2, median (range) | 71 (8 to >150) |
| VWF U/dL, median (range) | 203.0 (20.0-1046.0) |
| IQR (range) | 127.0-235.0 |
| ADAMTS-13 ng/mL, median (range) | 1044 (770-1600) |
Serum levels of VWF:Ag and ADAMTS-13:Ag and correlations with clinical characteristics
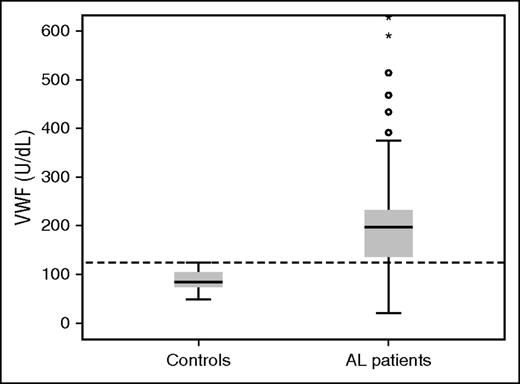
The median serum level of VWF:Ag in patients with AL amyloidosis was 203 U/dL (range 20-1046; IQR 127-235 U/dL) and was significantly higher than that measured in healthy controls (median 84.0 U/dL, range 48.0-124.0) (P < .001) (Figure 1). Importantly, 76% of patients with AL had VWF:Ag levels higher than the upper level measured in healthy controls (ie, >124.0 U/dL). There was no significant association of VWF:Ag levels with renal, cardiac, nerve, or liver involvement. There was no significant correlation of VWF:Ag levels with the levels of NTproBNP (or BNP) or Mayo stage, and there was no significant correlation with the degree of renal dysfunction. However, we found an inverse correlation of serum albumin levels with VWF:Ag levels (Spearman ρ −0.269, P = .006) and a positive correlation with the degree of proteinuria (Spearman ρ 0.204, P = .043). The levels of involved free light chains did not have any correlation with VWF:Ag levels and there was no correlation between VWF:Ag levels and the degree of infiltration of the bone marrow by plasma cells (Table 2). However, we found a correlation of VWF:Ag levels with lower systolic blood pressure (SBP) (<100 mm Hg).
Multivariate analysis for survival in 111 patients with AL amyloidosis
| . | P value . | HR . | 95% CI for HR . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| VWF ≥230.0 U/dL | .011 | 2.173 | 1.193 | 3.957 |
| SBP <100 mm Hg | .009 | 2.278 | 1.227 | 4.232 |
| Mayo stage I | 1 | |||
| Mayo stage II | .001 | 7.833 | 2.259 | 27.166 |
| Mayo stage III | <.001 | 15.078 | 4.247 | 53.533 |
| . | P value . | HR . | 95% CI for HR . | |
|---|---|---|---|---|
| Lower . | Upper . | |||
| VWF ≥230.0 U/dL | .011 | 2.173 | 1.193 | 3.957 |
| SBP <100 mm Hg | .009 | 2.278 | 1.227 | 4.232 |
| Mayo stage I | 1 | |||
| Mayo stage II | .001 | 7.833 | 2.259 | 27.166 |
| Mayo stage III | <.001 | 15.078 | 4.247 | 53.533 |
Median levels of ADAMTS-13:Ag were 1044 ng/mL (range 770-1600) and were not significantly different from the levels of healthy, age-matched controls (median 1170, range 745-1610) (P = .3). Importantly there was no correlation of the levels of ADAMTS-13:Ag with VWF:Ag (P > .5). However, ADAMTS-13:Ag levels had a significant inverse correlation with NTproBNP levels (r2 = 0.225, P < .001).
Prognostic importance of VWF:Ag and ADAMTS-13:Ag levels
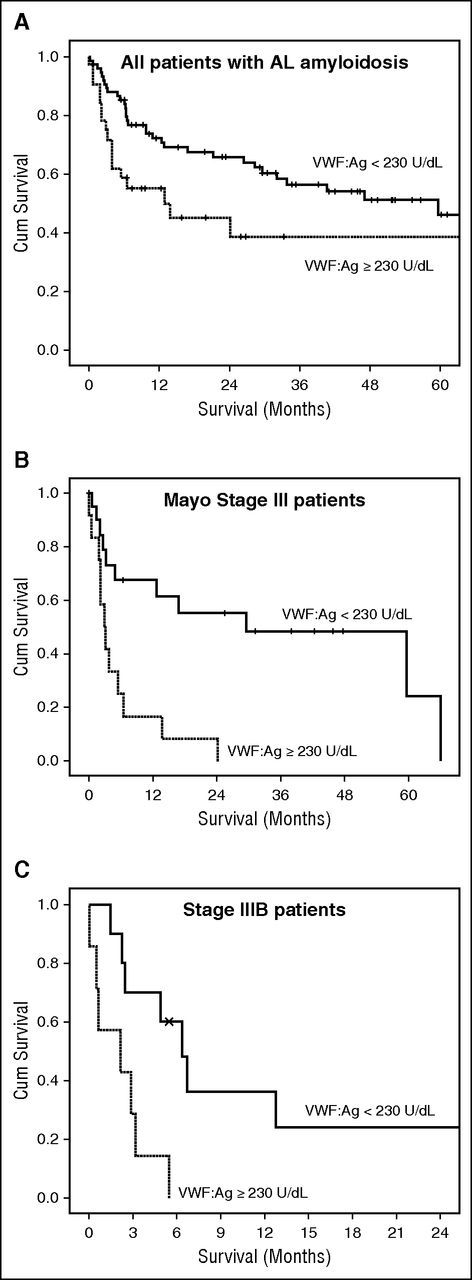
We then examined the potential prognostic significance of the levels of VWF:Ag. Because most patients had elevated levels of VWF:Ag, we initially evaluated the prognostic performance of the different quartiles and found that patients with VWF:Ag levels in the upper quartile had significantly inferior overall survival (median 14 months) compared with the middle quartiles (28 and 29 months) and the lower quartile (median 85 months) (P = .022). Because the survival curves indicated that patients with VWF:Ag ≥230.0 U/dL had a significant probability of early death, we also performed a receiver operating characteristic analysis to identify the level of VWF:Ag that was associated with early death. We found that a level of VWF:Ag ≥230.0 U/dL was associated with the higher probability of early death. This level was very similar to the cutoff of the upper quartile (ie, 235.0 U/dL). Thus, patients with VWF:Ag ≥230.0 U/dL had 3-month and 6-month mortality rates of 26% and 45%, respectively, vs 10% and 17% for patients with lower levels of VWF:Ag. Because the most important predictor of early death in patients with AL amyloidosis is cardiac involvement, we evaluated the prognostic importance of VWF:Ag after adjustment for Mayo stage. VWF levels ≥230.0 U/dL were independently associated with a higher risk of death (HR 2.17, 95% CI 1.2-3.95, P = .011) (Table 2). To further validate our results, we performed an internal validation by using bootstrapping of the Cox regression model, which confirmed the independent prognostic performance of VWF:Ag >230 U/dL and the validity of the model. The bootstrapped model is provided in supplemental Table 1, available on the Blood Web site. We also performed a multivariate analysis, adjusting for the levels of NTproBNP (either ≥4000 or ≥8500 pg/mL), which have been associated with very poor outcome. Similarly, VWF:Ag ≥230.0 U/dL was an independent predictor of death within 6 months from initiation of therapy. Low SBP has been identified as a predictor of poor outcome of patients with stage III disease; in the current analysis, this remained an independent predictor of high risk of early death. Because VWF was independently associated with outcome, patients with Mayo stage III disease had different outcomes according to VWF:Ag levels (Figure 2), so that those with VWF:Ag ≥230.0 U/dL had a very poor outcome (12-month survival of 17% vs 68% for patients with stage III and lower levels of VWF:Ag, respectively; P < .001). Patients with stage IIIB (stage III with NTproBNP ≥8500 pg/mL) had an extremely poor outcome. However, among patients with stage IIIB disease, those with VWF:Ag >230.0 U/dL had a significantly shorter survival (2 vs 6 months, P = .006; Figure 2). Thus, VWF could identify subsets of patients with very poor outcome even among stage III patients.
Survival of patients with VWF:Ag >230.0 U/dL. All patients (A), and patients with Mayo stage III (B), and stage IIIB disease (C).
Survival of patients with VWF:Ag >230.0 U/dL. All patients (A), and patients with Mayo stage III (B), and stage IIIB disease (C).
Discussion
To our knowledge, this is the first study to evaluate the prognostic significance of the serum levels of VWF:Ag in patients with AL amyloidosis. We found that the levels of VWF:Ag are extremely elevated in patients with AL amyloidosis compared with controls and, more importantly, have prognostic significance independent of the traditional cardiac biomarkers. Interestingly, VWF:Ag levels were not directly correlated with the degree of cardiac or renal dysfunction. However, addition of VWF:Ag levels could discriminate patients with poor prognosis even among stage III patients or among patients with stage IIIB. Thus, our data indicate that additional injury, beyond overt cardiac dysfunction, may also be implicated for the poor outcome of patients with advanced stage disease and that endothelium may be such an injured tissue.
Our aim was to evaluate the potential role of endothelial “dysfunction,” and VWF levels have been proposed as a surrogate marker for the evaluation of “injured” or “activated” endothelium.14,26 It has been documented that VWF:Ag is released by the ECs and that under pathologic conditions, VWF serves as a biomarker of endothelial damage and dysfunction14,26 and is involved in hemostasis and inflammatory activation.27,28 The specific mechanism of increased VWF:Ag in AL amyloidosis probably implicates ECs, because these are the most important producer of these molecules.
Several studies in patients with cardiovascular disease have shown that VWF levels are of prognostic significance.21,29-32 Patients with AL amyloidosis and heart involvement present mostly with preserved ejection fraction heart failure (HFpEF).33 Data in patients with non-amyloid–related HFpEF indicate that this entity is characterized by endothelial microvascular inflammation as a potential mediator, which may induce myocardial stiffening and increased collagen deposition, both recognized as underlying mechanisms of cardiac fibrosis and diastolic dysfunction.32 In addition, peripheral endothelial function has been show to be impaired in HFpEF and is further associated with an unfavorable prognosis.30,34 Thus, recently it was shown that in patients with HFpEF, VWF was an independent predictor of long-term outcome, independent of NTproBNP.31 The levels of VWF:Ag have also prognostic significance in other conditions, which may share some features in terms of hemodynamics with AL amyloidosis. Thus, in patients with cirrhosis, the levels of VWF:Ag were associated with hepatic venous pressure gradient, liver function, and clinical outcome.16 The association of VWF with low blood pressure is indicative of the potential role of endothelium as a major regulator of cardiovascular functional integrity. The fact that both low blood pressure and VWF remained independent prognostic factors is probably related to the fact that VWF reflects more than low blood pressure. Elevated levels of VWF have also been observed in other malignancies, including lymphoplasmacytic lymphoma/Waldenström macroglobulinemia, and have also been associated with outcome.35,36 However, in our patients, there was no strong correlation of the bone marrow infiltration by plasma cells or the levels of the involved free light chains with the levels of VWF:Ag.
We did not find any strong prognostic correlation of ADAMTS-13:Ag levels. Low levels of ADAMTS-13 have been considered as prognostic of cardiovascular complications in various settings,18,20,22,23 but we only found an inverse association with NTproBNP levels, in accordance with the previous studies. Although ADAMTS-13 is cleaving VWF multimers, there was no correlation with the levels of VWF, probably because the underlying mechanisms of VWF increase are different from the mechanism underlying thrombotic thrombocytopenic puprpura.17 Our study did not aim to measure the activity of VWF or ADAMTS-13, but only the levels of their antigens as markers of endothelial dysfunction or activation, and thus we cannot make any assumptions about the activity of these proteins in the plasma.
Our findings raise several questions regarding the pathophysiology underlying the increased levels of VWF:Ag. Preclinical evidence indicates that toxic light chains may directly cause microvascular dysfunction, and the injury caused by light chains may induce apoptotic injury to ECs.11,12 A more detailed functional study of the vascular function in patients with AL amyloidosis is required and, although some data have been published,13 our group is further investigating the functional and clinical consequences of AL amyloidosis in the arterial vessels.
The serum levels of VWF:Ag were able to identify patients at very high risk for death, independently of the other cardiac biomarkers, but our data alone cannot support the routine use of VWF as a prognostic marker without further validation. Importantly, the prognostic role of VWF was also seen in an independent study from the National Amyloidosis Center in London in patients with systemic AL amyloidosis, which showed that increased VWF levels were associated with shorter survival.37 Our results also indicate that further investigation is needed regarding the role and the implication of endothelium in the phenotype of the disease and the risk of disease-related complications (including early death).
In conclusion, VWF antigen levels are elevated in patients with AL amyloidosis, indicating that endothelial injury is present. However, VWF:Ag levels were not correlated with other features of the disease, such as pattern of organ involvement and cardiac biomarkers. For the first time, we found that high levels of VWF antigen are associated with a high risk of early death and shorter survival in patients with AL amyloidosis, independent of cardiac biomarkers. In addition, VWF antigen levels improve the prognostic ability of cardiac biomarkers in patients with AL amyloidosis, even among patients with stage III or stage IIIB disease. Our data also justify the investigation of the role of endothelial dysfunction in AL amyloidosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.K. designed the study, collected data, performed the statistical analysis, interpreted the data, and wrote the manuscript; I.P. designed the study, performed the measurements in the serum, and analyzed the data; E.T., M.R., M.G., E.E.-P., C.P., E. Psimenou, E.M., S.G., M.P., H.G., E. Papadopoulou, and K.S. collected data and reviewed the manuscript; A.K. and C.S. performed the measurements in the serum and analyzed the data; M.A.D. analyzed the data and cowrote the manuscript; and all coauthors critically reviewed the manuscript.
Conflict-of-interest disclosure: E.K. has received honoraria from Janssen, Takeda, and Amgen. E.T. has received honoraria from Medtronic, Celgene, Janssen, and Amgen. M.A.D. has received honoraria from Celgene, Janssen, Takeda, and Amgen.
Correspondence: Efstathios Kastritis, Department of Clinical Therapeutics, National and Kapodistrian University of Athens School of Medicine, 80 Vas. Sofias Ave, 115 28 Athens, Greece; e-mail: ekastritis@gmail.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal