Key Points
The reversible association of deoxyHb with band 3 acts as an O2-triggered molecular switch to regulate erythrocyte properties.
Transgenic mice lacking the deoxyHb site on band 3 fail to respond to changes in O2 with changes in erythrocyte properties.
Abstract
Functional studies have shown that the oxygenation state of the erythrocyte regulates many important pathways, including glucose metabolism, membrane mechanical stability, and cellular adenosine triphosphate (ATP) release. Deoxyhemoglobin (deoxyHb), but not oxyhemoglobin, binds avidly and reversibly to band 3, the major erythrocyte membrane protein. Because band 3 associates with multiple metabolic, solute transport, signal transduction, and structural proteins, the hypothesis naturally arises that the O2-dependent regulation of erythrocyte properties might be mediated by the reversible association of deoxyHb with band 3. To explore whether the band 3–deoxyHb interaction constitutes a “molecular switch” for regulating erythrocyte biology, we have generated transgenic mice with mutations in the deoxyHb-binding domain of band 3. One strain of mouse contains a “humanized” band 3 in which the N-terminal 45 residues of mouse band 3 are replaced by the homologous sequence from human band 3, including the normal human band 3 deoxyHb-binding site. The second mouse contains the same substitution as the first, except the deoxyHb site on band 3 (residues 12-23) has been deleted. Comparison of these animals with wild-type mice demonstrates that the following erythrocyte properties are controlled by the O2-dependent association of hemoglobin with band 3: (1) assembly of a glycolytic enzyme complex on the erythrocyte membrane which is associated with a shift in glucose metabolism between the pentose phosphate pathway and glycolysis, (2) interaction of ankyrin with band 3 and the concomitant regulation of erythrocyte membrane stability, and (3) release of ATP from the red cell which has been linked to vasodilation.
Introduction
Although red blood cells (RBCs) were once thought to be insensitive to molecular signals, they now are known to communicate sensitively with their environment, serving as both recipients and producers of extracellular stimuli.1-3 Erythrocyte components that engage in this signaling with other cell types include nitric oxide,4 adenosine triphosphate (ATP)/adenosine diphosphate (ADP),5 adenosine,6 CD47,7 complement receptors (eg, CD598 and CR19 ), cytokine receptors (eg, Duffy antigen10 ), and a large variety of ectoenzymes (eg, endothelin endopeptidase,11 cyclic ADP ribose hydrolase,12 adenosine deaminase,13 and acetylcholinesterase14 ). Signaling molecules from other cell types that modulate erythrocyte function include prostaglandins,15 β-adrenergic agonists,16 insulin,17 C-peptide,18 transforming growth factor β1,19 and endothelin-1.20
Among the most prominent signaling molecules to regulate erythrocyte properties is diatomic oxygen (O2), which is abundant in the lungs but present at low concentrations in the tissues. We have previously shown that as O2 levels change, the association of glycolytic enzymes (GEs) with binding sites on band 3 (the most abundant polypeptide in the human erythrocyte membrane) also changes, leading to a shift in glucose consumption from glycolysis at low O2 levels to the pentose phosphate pathway at higher O2 levels.21,22 This shunting of glucose into the pentose phosphate pathway drives production of reduced nicotinamide adenine dinucleotide phosphate, a metabolite that protects the RBC against the oxidative stress that accompanies increased levels of O2. We have also shown that elevation of O2 promotes the strengthening of band 3–ankyrin interactions, thereby stabilizing the erythrocyte membrane during its turbulent flow from the lungs into the capillary beds.23,24 Hypoxia, in contrast, leads to rupture of this important membrane-to-cytoskeleton bridge, enabling the deoxygenated RBCs to move more efficiently through narrow capillaries during transit back to the lungs.25 Finally, deoxygenation has been shown to promote ATP release from erythrocytes, leading to activation of P2Y receptors on endothelial cells and the consequent vasodilation that can also facilitate blood flow from hypoxic tissues back to the lungs.26,27
In the search for an O2-regulated molecular switch that can account for the O2 modulation of RBC properties, the O2-dependent band 3–hemoglobin (Hb) interaction has emerged as a prominent candidate.1,28-30 Evidence that Hb associates reversibly with band 3 as O2 pressures change arises from several observations. First, ultrafiltration assays have shown that deoxyhemoglobin (deoxyHb) binds to erythrocyte membranes with eightfold greater affinity than oxyhemoglobin (oxyHb).31 Second, a cocrystal structure of deoxyHb with the N terminus of band 3 reveals that the N terminus of band 3 binds deep within the central cavity of the deoxyHb tetramer.32 This cavity is inaccessible upon Hb oxygenation.33 Third, an immobilized preparation of the cytoplasmic domain of band 3 (cdb3) has been found to bind deoxyHb, but not oxyHb, at physiological pH and ionic strength.34 Fourth, fluorescence resonance energy quenching of a fluorophore on band 3 by Hb has been shown to be greatly enhanced by Hb deoxygenation.35 Finally, the Hb-O2 saturation curve is shifted strongly to the right (higher O2 pressures) in the presence of cdb328,32 ; that is, an observation that requires deoxyHb to bind preferentially to band 3. The band 3–Hb association activities described above are reversible and occur over the range of O2 pressures that the RBC experiences in vivo.28 Collectively, these data suggest that the deoxyHb–band 3 interaction is a logical candidate for the O2-triggered molecular switch that controls RBC properties. The data further predict that erythrocytes lacking a deoxyHb-binding site should display little sensitivity to O2 compared with normal erythrocytes.
To test this hypothesis, we generated transgenic mice in which the normal human deoxyHb-binding site28,35 was inserted into the mouse Slc4a1 (the gene that encodes band 3) locus to create a “humanized” mouse with a hybrid human-murine band 3. We also inserted a mutant human sequence with the deoxyHb-binding site deleted into the same locus to generate transgenic mice lacking a deoxyHb-binding site. Analysis of the usual O2-dependent properties of erythrocytes from these mice confirm our hypothesis in most major respects, with regulation of membrane assembly of the glycolytic enzyme complex and control of band 3–cytoskeletal interactions abolished in mice lacking the deoxyHb-binding site, and with O2-dependent ATP release substantially suppressed in the same transgenic mice.
Materials and methods
Generation of transgenic mice by homologous recombination in embryonic stem cells
Standard techniques were used to generate transgenic mice expressing either a “humanized” band 3 with the human deoxyHb-binding site, or a band 3 lacking a deoxyHb-binding site. The homologous recombination strategy used to target the murine band 3 (Slc4A1) allele is shown in Figure 1. Each strain was backcrossed to C56BL/6 for at least 7 generations. Detailed protocols are included in supplemental Materials and methods (available on the Blood Web site).
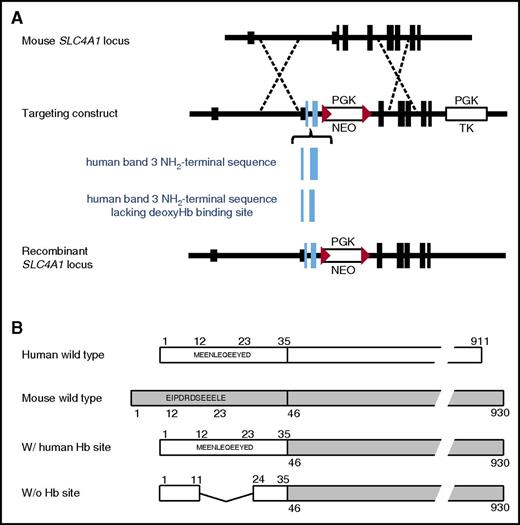
Design of transgenic mice with altered deoxyHb-binding sites on erythrocyte band 3. (A) The homologous recombination strategy to targeting the murine band 3 (Slc4A1) allele. The blue boxes represent the exons of human SLC4A1 knocked into the Slc4A1 locus. The deletion of the sequence encoding amino acids 12-23 is indicated by a smaller box. The red triangles represent loxP sites which flank a PGK-NEO gene to facilitate subsequent removal. (B) Schematic representation of erythrocyte band 3 proteins from normal human, wild-type mouse, and the 2 transgenic mice including 1 with humanized band 3 (w/ human Hb site) and the other lacking the deoxyHb-binding site on band 3 (w/o Hb site). w/, with; w/o, without.
Design of transgenic mice with altered deoxyHb-binding sites on erythrocyte band 3. (A) The homologous recombination strategy to targeting the murine band 3 (Slc4A1) allele. The blue boxes represent the exons of human SLC4A1 knocked into the Slc4A1 locus. The deletion of the sequence encoding amino acids 12-23 is indicated by a smaller box. The red triangles represent loxP sites which flank a PGK-NEO gene to facilitate subsequent removal. (B) Schematic representation of erythrocyte band 3 proteins from normal human, wild-type mouse, and the 2 transgenic mice including 1 with humanized band 3 (w/ human Hb site) and the other lacking the deoxyHb-binding site on band 3 (w/o Hb site). w/, with; w/o, without.
Blood analysis
Blood was collected by retro-orbital bleeding from 6- to 10-week-old mice. Complete blood counts were performed on an automatic hematology analyzer (Siemens ADVIA 120 Hematology System) following the manufacturer’s instructions. Reticulocyte counts were determined using Syto RNASelect green fluorescent cell stain (Invitrogen). Osmotic fragility was determined by measuring hemolysis of erythrocytes placed in sodium chloride solutions of varying concentrations as described previously.36 Cells isolated from bone marrow or spleen were stained with anti-ter119 antibody and evaluated by fluorescence-activated cell sorter (FACS; BD Bioscience). RBC deformability was measured by ektacytometry, as described previously.37 Scanning flow cytometry analysis of murine RBCs was performed as described in van Beers et al.38
Analysis of Hb binding to KI-IOVs of transgenic murine RBCs
Potassium iodide–stripped inside out vesicles (KI-IOVs) were prepared as described previously.25,39,40 Murine Hb was prepared from blood stored at 4°C for 2 days to deplete 2,3-bisphosphoglycerate. KI-IOVs and Hb were mixed in either oxygenated or deoxygenated 10 mM bis-Tris/acetate buffer (pH 6.0) in a septum-capped tube. The deoxygenated sample was then further deoxygenated by flowing argon across the surface of the suspension, using a syringe that penetrated the septum to introduce the argon and a separate syringe to vent the excess argon. After incubation of each sample at room temperature for 30 minutes, the KI-IOV suspension was pelleted in the sealed tube at 30 000g for 30 minutes. The supernatant was removed and the bound Hb in the KI-IOV pellet was eluted with phosphate-buffered saline (PBS; pH 7.4) and quantitated by measuring its absorbance at 540 nm. KI-IOV concentrations were measured by microBCA protein assay (Thermo Fisher). To calculate deoxyHb binding to the deoxyHb-binding site on band 3, Hb binding to KI-IOVs under oxygenated conditions was subtracted from deoxyHb binding under deoxygenated conditions, and 50% of the KI-IOV protein was assumed to be band 3.40,41
Erythrocyte immunofluorescent staining
Immunofluorescent staining of intact oxygenated or deoxygenated erythrocytes for glycolytic enzymes, glucose-6-phosphate dehydrogenase (G6PDH), ankyrin, or band 3 was performed as described previously.42 Detailed protocols are included in supplemental Materials and methods.
Analysis of effect of O2 on band 3 retention in detergent-extracted membrane cytoskeletons
Detergent-extracted cytoskeletons were prepared as described previously.25 In brief, oxygenated or deoxygenated erythrocytes were solubilized in septum-sealed tubes in an equal volume of preoxygenated or deoxygenated PBS with 2% Triton X-100. The insoluble membrane cytoskeletal fraction was isolated by centrifugation at 25 000g for 30 minutes at 4°C, dissolved in 2% sodium dodecyl sulfate (SDS) and then separated by SDS–polyacrylamide gel electrophoresis (PAGE). Band 3 in the cytoskeletal pellets was quantified by western blotting using anti-cdb3 and anti-actin polyclonal antibodies, respectively. Densitometric analysis of band 3 in the cytoskeletal pellet was normalized using actin as a loading control.
Analysis of effect of O2 on erythrocyte ATP release
RBCs were prepared as described previously.43 Washed RBCs (10 μL) were counted and hemolyzed in distilled deionized H2O (ddH2O), and the intracellular ATP content was measured using a CellTiter-Glo Luminescent Cell Viability Assay kit (Promega). Pure ATP dissolved in the same buffer was used to construct a standard curve. Washed RBCs were also either oxygenated or deoxygenated for 8 minutes in septum-sealed tubes as described in the supplemental Materials and methods, then incubated at room temperature for 30 minutes with occasional gentle mixing. After mild centrifugation at 500g for 5 minutes to pellet the RBCs, the supernatant was removed to measure both ATP and Hb content. The pelleted RBCs were then lysed with ddH2O and the Hb concentration was measured to calculate erythrocyte numbers. The number of spontaneously hemolyzed RBCs was calculated based on the absorbance of the supernatant and the Hb content of RBCs of each strain. Samples were only included if Hb measurements in supernatant indicated that <0.1% of RBCs had hemolyzed. The amount of released ATP corresponding to this level of hemolysis was calculated by using the intracellular ATP content of RBCs in each strain. ATP arising from hemolysis was subtracted from the total ATP in supernatant to yield the net ATP released from intact RBCs.
Results
Characterization of transgenic murine erythrocytes with altered deoxyHb-binding sites on band 3
To determine whether the reversible binding of deoxyHb to band 3 constitutes the molecular switch responsible for O2 regulation of erythrocyte properties, 2 transgenic mice with altered band 3 sequences were generated by homologous recombination in embryonic stem cells (Figure 1A). In 1 mouse, residues 1 to 45 of murine band 3 (which includes the deoxyHb-binding site) were replaced with the homologous sequence from human band 3 (residues 1-35).28 The resulting mouse expressed a “humanized” band 3 with the human deoxyHb-binding site. The second transgenic mouse contained the same human sequence insert, except that the human deoxyHb-binding residues (residues 12-23)35 were deleted (Figure 1B). These mice allowed comparison of the O2-regulated properties in RBCs from mice with (1) a normal murine deoxyHb-binding site, (2) a normal human deoxyHb-binding site, or (3) no deoxyHb-binding site.
To verify the presence of the human NH2-terminal band 3 sequence in transgenic erythrocytes, a monoclonal antibody recognizing residues 1 to 10 of human (but not mouse) band 3 was used to immunostain the transgenic erythrocytes. As shown in Figure 2A, the antibody failed to detect band 3 in RBCs from wild-type mice, whereas erythrocytes from both lines of transgenic mice displayed the anticipated membrane-localized staining. Moreover, as shown in supplemental Figure 1, band 3 from mice containing either the human deoxyHb-binding site (7 amino acids shorter than the wild-type mouse band 3) or no deoxyHb-binding site (20 amino acids shorter than wild-type mouse band 3) migrated faster on SDS-polyacrylamide gels than wild-type mouse band 3. These data demonstrate that the expected human band 3 sequences were successfully inserted into the transgenic mice.
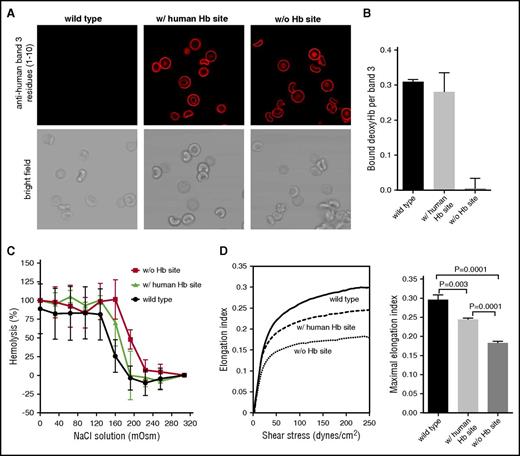
Characterization of transgenic mouse erythrocytes with altered deoxyHb-binding sites on band 3. (A) Confocal immunofluorescence (top) and corresponding brightfield (bottom) images of murine RBCs stained with a monoclonal antibody to the NH2 terminus (residues 1-10) of human band 3. Note that wild-type mouse RBCs do not stain whereas both transgenic erythrocytes do. (B) Binding of deoxygenated murine Hb to KI-IOVs of normal and transgenic mouse erythrocytes. The y-axis shows the molecular ratio of deoxyHb to band 3. (C) Comparison of the osmotic fragility of wild-type and transgenic mouse erythrocytes. (D) Comparison of the deformability of wild-type and transgenic mouse erythrocytes by ektacytometry. The elongation index upon subjection of the erythrocytes to increasing shear stress is shown. The mean of maximal elongation index of 3 experiments is also shown.
Characterization of transgenic mouse erythrocytes with altered deoxyHb-binding sites on band 3. (A) Confocal immunofluorescence (top) and corresponding brightfield (bottom) images of murine RBCs stained with a monoclonal antibody to the NH2 terminus (residues 1-10) of human band 3. Note that wild-type mouse RBCs do not stain whereas both transgenic erythrocytes do. (B) Binding of deoxygenated murine Hb to KI-IOVs of normal and transgenic mouse erythrocytes. The y-axis shows the molecular ratio of deoxyHb to band 3. (C) Comparison of the osmotic fragility of wild-type and transgenic mouse erythrocytes. (D) Comparison of the deformability of wild-type and transgenic mouse erythrocytes by ektacytometry. The elongation index upon subjection of the erythrocytes to increasing shear stress is shown. The mean of maximal elongation index of 3 experiments is also shown.
To investigate whether the modified band 3 sequences altered deoxyHb–band 3 interactions, we prepared KI-IOVs from the mutated erythrocytes and examined their ability to bind murine Hb. As shown in Figure 2B, KI-IOVs containing the human deoxyHb site on band 3 showed similar deoxyHb-binding properties as wild-type murine KI-IOVs, whereas KI-IOVs from erythrocytes lacking the deoxy Hb-binding site showed no specific deoxyHb binding. These results demonstrate that transgenic RBCs containing the normal or mutant human band 3 deoxyHb-binding sequences can be used to investigate the role of deoxyHb binding to band 3 in O2 regulation of erythrocyte properties.
Complete blood counts demonstrated that mice containing the intact human deoxyHb-binding site exhibited essentially normal indices. In contrast, mice lacking the deoxyHb-binding site displayed changes in RBC indices. The RBC count was significantly reduced with a concomitant decrease in the hematocrit and Hb levels, as well as increases in the mean corpuscular volume (MCV), mean corpuscular Hb (MCH), the number of ter119+ cells in both bone marrow and spleen, and increased spleen weights compared with control animals (Table 1). White blood cell (WBC) counts were also elevated in the animals lacking the deoxyHb-binding site. However, because the MCV, Hb, MCH, and WBC values all fell within the 95% confidence interval for control mice, we did not investigate these changes further. Despite the increased MCV measured in the complete blood count, the morphology of the RBCs from wild-type mice and the RBCs containing the normal or mutant human band 3 deoxyHb-binding sequences were similar under both oxygenated and deoxygenated conditions by standard confocal microscopy as well as when analyzed by scanning flow cytometry (Figure 2A; supplemental Figure 6). Transgenic mice lacking the deoxyHb-binding site displayed increased osmotic fragilities (Figure 2C) and decreased mechanical deformabilities (Figure 2D). Consistent with these data, we also observed dark pigmentation of the spleen, indicating increased erythrocyte turnover. We conclude that erythrocytes from the transgenic mice lacking the deoxyHb-binding site undergo some hemolysis, which could derive from the increased osmotic fragility shown in Figure 2C. To compensate for this enhanced RBC turnover, the mutant mice have an increased percentage of ter119+ erythroid cells in the marrow and spleen (Table 1).
Transgenic murine hematologic indices
| . | Mean ± SD . | ||
|---|---|---|---|
| Wild-type . | w/ human Hb site . | w/o Hb site . | |
| n | 8 | 12 | 15 |
| (4 female; 4 male) | (6 female; 6 male) | (7 female; 8 male) | |
| RBC, ×1012/L | 10.67 ± 0.64 | 10.58 ± 0.25 | 8.91 ± 1.36*† |
| Hb, g/L | 160.3 ± 2.8 | 159.8 ± 5.1 | 153.7 ± 6.1*† |
| Hematocrit, % | 47.34 ± 2.04 | 44.63 ± 1.40* | 44.06 ± 2.46* |
| MCH | 15.33 ± 0.39 | 15.36 ± 0.42 | 16.77 ± 0.80* |
| MCV, fL | 42.18 ± 1.69 | 42.12 ± 0.48 | 47.68 ± 2.27* |
| Reticulocytes, % | 4.35 ± 0.29 | 4.62 ± 0.53 | 5.14 ± 0.78 |
| WBC, ×109/L | 7.00 ± 3.36 | 7.68 ± 0.93* | 11.86 ± 3.46*† |
| Platelet, ×109/L | 899.8 ± 110 | 850.0 ± 104 | 805.4 ± 111 |
| n | 5 | 4 | 5 |
| Total cells in marrow, ×107 | 7.30 ± 0.62 | 8.38 ± 1.84 | 7.78 ± 1.65 |
| Ter119+ cells in marrow, ×107 | 2.87 ± 0.39 | 3.43 ± 1.15 | 3.63 ± 1.07 |
| Ter119+ cells in marrow (%) | 39.3 ± 4.2 | 40.8 ± 10.5 | 46.6 ± 8.1*† |
| Total cells in spleen, ×107 | 30.14 ± 5.81 | 34.35 ± 7.37 | 60.61 ± 9.38*† |
| Ter119+ cells in spleen, ×107 | 14.88 ± 2.83 | 19.98 ± 5.55 | 50.49 ± 12.95*† |
| Ter119+ cells in spleen, % | 49.9 ± 3.1 | 57.8 ± 6.4* | 82.4 ± 12.4*† |
| Spleen weight, g | 0.11 ± 0.01 | 0.13 ± 0.04 | 0.24 ± 0.04*† |
| . | Mean ± SD . | ||
|---|---|---|---|
| Wild-type . | w/ human Hb site . | w/o Hb site . | |
| n | 8 | 12 | 15 |
| (4 female; 4 male) | (6 female; 6 male) | (7 female; 8 male) | |
| RBC, ×1012/L | 10.67 ± 0.64 | 10.58 ± 0.25 | 8.91 ± 1.36*† |
| Hb, g/L | 160.3 ± 2.8 | 159.8 ± 5.1 | 153.7 ± 6.1*† |
| Hematocrit, % | 47.34 ± 2.04 | 44.63 ± 1.40* | 44.06 ± 2.46* |
| MCH | 15.33 ± 0.39 | 15.36 ± 0.42 | 16.77 ± 0.80* |
| MCV, fL | 42.18 ± 1.69 | 42.12 ± 0.48 | 47.68 ± 2.27* |
| Reticulocytes, % | 4.35 ± 0.29 | 4.62 ± 0.53 | 5.14 ± 0.78 |
| WBC, ×109/L | 7.00 ± 3.36 | 7.68 ± 0.93* | 11.86 ± 3.46*† |
| Platelet, ×109/L | 899.8 ± 110 | 850.0 ± 104 | 805.4 ± 111 |
| n | 5 | 4 | 5 |
| Total cells in marrow, ×107 | 7.30 ± 0.62 | 8.38 ± 1.84 | 7.78 ± 1.65 |
| Ter119+ cells in marrow, ×107 | 2.87 ± 0.39 | 3.43 ± 1.15 | 3.63 ± 1.07 |
| Ter119+ cells in marrow (%) | 39.3 ± 4.2 | 40.8 ± 10.5 | 46.6 ± 8.1*† |
| Total cells in spleen, ×107 | 30.14 ± 5.81 | 34.35 ± 7.37 | 60.61 ± 9.38*† |
| Ter119+ cells in spleen, ×107 | 14.88 ± 2.83 | 19.98 ± 5.55 | 50.49 ± 12.95*† |
| Ter119+ cells in spleen, % | 49.9 ± 3.1 | 57.8 ± 6.4* | 82.4 ± 12.4*† |
| Spleen weight, g | 0.11 ± 0.01 | 0.13 ± 0.04 | 0.24 ± 0.04*† |
SD, standard deviation; w/, with; w/o, without.
P < .05 compared with the wild-type (C57BL/6) strain.
P < .05 compared with the transgenic strain with the human Hb site.
O2 regulation of GE assembly on the erythrocyte membrane of transgenic mice
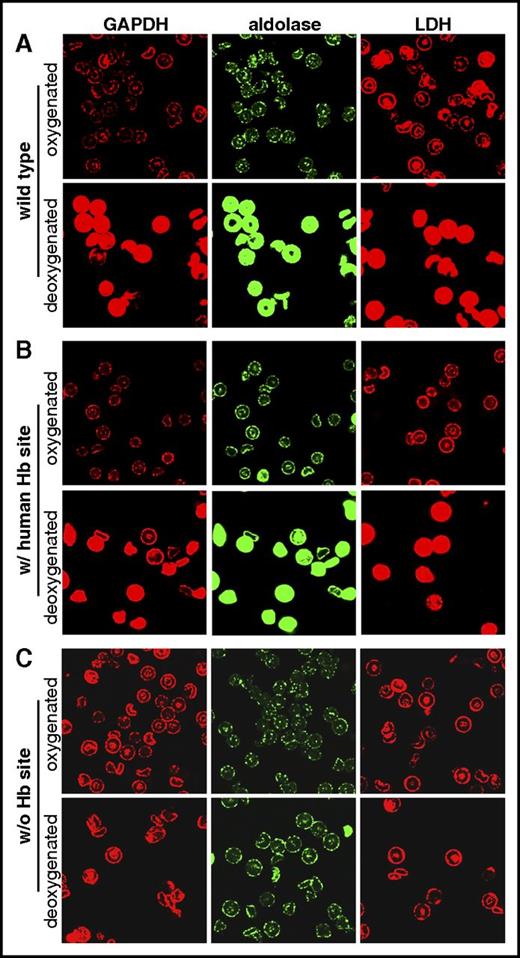
GEs have been shown to organize into multienzyme complexes on band 3 in both healthy human and murine erythrocytes.42,44 These complexes rapidly dissociate and release into the cytosol upon RBC deoxygenation in a manner that correlates with a shift in glucose consumption from the pentose phosphate pathway (oxygenated conditions) to glycolysis (deoxygenated conditions).21,22 To determine whether this O2-dependent GE dissociation is observed in the humanized transgenic erythrocytes, we fixed oxygenated or deoxygenated erythrocytes and stained them with antibodies to 3 different GEs. As shown in Figure 3, all 3 GEs examined (ie, glyceraldehyde-3-phosphate dehydrogenase [GADPH], aldolase, and lactate dehydrogenase [LDH]) were membrane-localized in the oxygenated cells, including those lacking the deoxyHb site. However, upon deoxygenation, GEs in wild-type erythrocytes and erythrocytes containing the human deoxyHb-binding site (with human Hb site) were released into the cytoplasm in ∼90% and 70% of the RBCs, respectively, whereas GEs from mice lacking the deoxyHb-binding site (without Hb site) showed <5% GE displacement. We also evaluated the stained erythrocytes by FACS. As shown in supplemental Figure 3, a right shift in the fluorescence intensity of the deoxygenated RBCs containing either the murine or human deoxyHb-binding site reveals greater GE displacement in deoxygenated cells containing a deoxyHb site than cells missing this site. We conclude that the reversible binding of deoxyHb to band 3 regulates the O2-dependent assembly of a GE complex on the membrane.
Effect of deoxygenation on the localization of GEs on the membranes of wild-type and transgenic murine erythrocytes. Erythrocytes from (A) wild-type, (B) transgenic mice containing a human deoxyHb-binding site (w/ human Hb site), or (C) transgenic mice lacking a deoxyHb-binding site (w/o Hb site) were either oxygenated or deoxygenated prior to fixation and staining with antibodies against the indicated GEs.
Effect of deoxygenation on the localization of GEs on the membranes of wild-type and transgenic murine erythrocytes. Erythrocytes from (A) wild-type, (B) transgenic mice containing a human deoxyHb-binding site (w/ human Hb site), or (C) transgenic mice lacking a deoxyHb-binding site (w/o Hb site) were either oxygenated or deoxygenated prior to fixation and staining with antibodies against the indicated GEs.
Brief examination of the disposition of the key regulatory enzyme in the pentose phosphate pathway, G6PDH, upon RBC deoxygenation shows similar enzyme displacement during erythrocyte deoxygenation (supplemental Figure 4). However, in contrast to the behavior of glycolytic enzymes, G6PDH displays a clear punctate distribution in the cytosol of deoxygenated RBCs containing a deoxyHb-binding site. These data would suggest that G6PDH remains assembled in a macromolecular complex upon displacement from the membrane, that is, in contrast to glycolytic enzymes which all show a smooth cytoplasmic distribution upon deoxygenation. Investigation of how this different behavior affects the function and biochemistry of G6PDH and the pentose phosphate pathway will require significant additional studies. However, the observed translocation of the G6PDH complex upon deoxygenation is consistent with earlier data from our laboratory and others21,22 showing that glucose flux through the pentose phosphate pathway is partially inhibited upon RBC deoxygenation. Moreover, the absence of any G6PDH displacement in RBCs lacking the deoxyHb-binding site confirms the role of the reversible binding of deoxyHb to band 3 in this O2-regulated pathway.
O2 regulation of the band 3–ankyrin bridge in erythrocyte membranes of transgenic mice
The band 3–ankyrin interaction forms 1 of 2 major bridges linking the erythrocyte membrane to its spectrin/actin-based cytoskeleton.25,45 Although 77% of band 3 in oxygenated erythrocytes is immobilized by association with the spectrin cytoskeleton, only ∼35% of band 3 remains similarly attached in deoxygenated cells,25 suggesting that O2 can regulate membrane structural properties. To explore whether O2 modulates the ankyrin bridge via its control of band 3-Hb interactions, we isolated cytoskeletons from wild-type or transgenic mouse erythrocytes by plunging them into 1% Triton X-100 solutions under both oxygenated and deoxygenated conditions. The detergent-insoluble cytoskeletons were then isolated and analyzed for band 3 content by western blotting. As seen in Figure 4, band 3 retention in cytoskeletons from deoxygenated RBCs containing either the murine or human deoxyHb site was 40% or 30% lower than in cytoskeletons prepared from oxygenated RBCs, respectively. In contrast, band 3 retention in cytoskeletons from murine erythrocytes lacking the deoxyHb-binding site was unaffected by O2 pressure (Figure 4). These data demonstrate that the band 3–ankyrin bridge is modulated by the O2-dependent interaction of deoxyHb with band 3.
Effect of deoxygenation on band 3 retention in membrane cytoskeletons isolated from wild-type and transgenic mouse erythrocytes. (A) Erythrocytes from wild-type (left panel), transgenic mice containing a human deoxyHb-binding site (middle panel), or transgenic mice lacking a deoxyHb-binding site (right panel) were either oxygenated or deoxygenated prior to solubilization in 1% Triton X-100. Detergent-insoluble membrane fractions containing the spectrin/actin membrane skeletons were separated by SDS-PAGE and immunoblotted with anti-cdb3 or antiactin antibodies. (B) Densitometric analysis of the relative amount of band 3 retained in the above cytoskeletal pellets using actin as a loading control. All values of band 3 retention in cytoskeletons derived from deoxygenated RBCs are relative to the amount of band 3 retained in the pellet of the corresponding oxygenated erythrocytes (mean of 3 experiments ± standard deviation [SD]).
Effect of deoxygenation on band 3 retention in membrane cytoskeletons isolated from wild-type and transgenic mouse erythrocytes. (A) Erythrocytes from wild-type (left panel), transgenic mice containing a human deoxyHb-binding site (middle panel), or transgenic mice lacking a deoxyHb-binding site (right panel) were either oxygenated or deoxygenated prior to solubilization in 1% Triton X-100. Detergent-insoluble membrane fractions containing the spectrin/actin membrane skeletons were separated by SDS-PAGE and immunoblotted with anti-cdb3 or antiactin antibodies. (B) Densitometric analysis of the relative amount of band 3 retained in the above cytoskeletal pellets using actin as a loading control. All values of band 3 retention in cytoskeletons derived from deoxygenated RBCs are relative to the amount of band 3 retained in the pellet of the corresponding oxygenated erythrocytes (mean of 3 experiments ± standard deviation [SD]).
A second method to evaluate the status of the band 3–ankyrin bridge in whole cells is to measure the accessibility of ankyrin epitopes to antiankyrin antibodies in intact cells. As shown previously,25 ankyrin epitopes are significantly less accessible to antibodies when the ankyrin–band 3 bridge is intact than when it is dissociated. As shown in Figure 5 and supplemental Figure 5, antiankyrin immunostaining under oxygenated conditions is weak, regardless of the presence or absence of a deoxyHb-binding site on band 3; that is, confirming that most ankyrin epitopes are occluded in fully oxygenated RBCs. In contrast, when the same cells are deoxygenated, a significant increase in ankyrin immunostaining is seen in both wild-type and transgenic RBCs containing a deoxyHb-binding site, but not in transgenic RBCs lacking this site. Importantly, these conclusions are confirmed by flow cytometry analysis of parallel preparations of the same cells, where a right shift in the fluorescence intensity of the deoxygenated RBCs reveals greater antiankyrin accessibility in deoxygenated cells containing a deoxyHb site than cells missing this site. We conclude that O2-dependent control of the band 3–ankyrin bridge is governed by the reversible association of deoxyHb with band 3.
Effect of deoxygenation on the accessibility of ankyrin epitopes in transgenic murine erythrocytes evaluated by both immunofluorescence staining and flow cytometry. (A) RBCs were oxygenated or deoxygenated, then fixed and stained with a monoclonal antibody to ankyrin. The stained RBCs were observed using a confocal fluorescence microscope (top) and further analyzed for antiankyrin fluorescence staining intensity by flow cytometry (bottom). (B) Mean fluorescence intensity (MFI) of antiankyrin staining in oxygenated and deoxygenated erythrocytes. The relative MFI of oxygenated erythrocytes was set at 1 and compared with the MFI of the deoxygenated RBCs (mean of 3 experiments ± SD).
Effect of deoxygenation on the accessibility of ankyrin epitopes in transgenic murine erythrocytes evaluated by both immunofluorescence staining and flow cytometry. (A) RBCs were oxygenated or deoxygenated, then fixed and stained with a monoclonal antibody to ankyrin. The stained RBCs were observed using a confocal fluorescence microscope (top) and further analyzed for antiankyrin fluorescence staining intensity by flow cytometry (bottom). (B) Mean fluorescence intensity (MFI) of antiankyrin staining in oxygenated and deoxygenated erythrocytes. The relative MFI of oxygenated erythrocytes was set at 1 and compared with the MFI of the deoxygenated RBCs (mean of 3 experiments ± SD).
O2 regulation of ATP release from erythrocytes of wild-type and transgenic mice
Recent reports have shown that deoxygenation stimulates ATP release from human erythrocytes.26 In order to determine whether the reversible deoxyHb–band 3 association plays a role in this O2 regulation, we studied O2-dependent ATP release from both wild-type and transgenic red cells. For this purpose, oxygenated or deoxygenated RBCs were pelleted gently at 400g and analyzed for ATP released into the supernatant. To avoid the contamination from ATP derived from RBC hemolysis, the Hb concentration in the supernatant was also measured and the corresponding amount of released ATP was subtracted from the total supernatant ATP. To accurately evaluate the amount of ATP that would have been released during hemolysis of each RBC type, we also measured the intracellular ATP for each transgenic erythrocyte and found RBCs with the murine deoxyHb-binding site (2.9 ± 0.5 nmol/108 RBCs), the human deoxyHb-binding site (3.5 ± 0.5 nmol/108 RBCs), and no deoxyHb site (3.3 ± 0.5 nmol/108 RBCs) to contain roughly similar levels of ATP. Based on these measurements, the net ATP released from deoxygenated wild-type mouse erythrocytes was ∼1.5-fold higher than from oxygenated wild-type RBCs, that is, a value somewhat lower than previously reported.26 Similarly, ATP levels in the supernatants of deoxygenated transgenic mouse erythrocytes that contain the human deoxyHb site (with human Hb site) were ∼1.4 times greater under deoxygenated than in oxygenated conditions. In contrast, O2-dependent ATP release was not statistically significant in murine RBCs lacking a deoxyHb-binding site (without Hb site) (Figure 6). The suppression of O2-dependent ATP release in RBCs lacking a deoxyHb-binding site argues that the reversible association of deoxyHb with band 3 may contribute to O2 regulation of ATP release.
Effect of deoxygenation on ATP release from wild-type and transgenic mouse erythrocytes. Washed RBCs were oxygenated or deoxygenated and kept for 30 minutes at room temperature, and gently pelleted. The supernatant was collected and evaluated for ATP and Hb content. The pelleted RBCs were then lysed with ddH2O and the Hb concentration was measured to calculate erythrocyte numbers. ATP arising from hemolysis was subtracted from the total ATP in supernatant to yield net ATP released by deoxygenation. Absolute numbers of ATP released from intact erythrocytes containing the murine deoxyHb-binding site (wild-type), the human deoxyHb-binding site (w/ human Hb site), and no deoxyHb site (w/o Hb site) in oxygenated vs deoxygenated condition are 44 ± 4.0 pmol/108 cells vs 67 ± 5.1 pmol/108 cells, 39 ± 3.1 pmol/108 cells vs 54 ± 1.4 pmol/108 cells, and 53 ± 1.5 pmol/108 cells vs 60 ± 3.0 pmol/108 cells.
Effect of deoxygenation on ATP release from wild-type and transgenic mouse erythrocytes. Washed RBCs were oxygenated or deoxygenated and kept for 30 minutes at room temperature, and gently pelleted. The supernatant was collected and evaluated for ATP and Hb content. The pelleted RBCs were then lysed with ddH2O and the Hb concentration was measured to calculate erythrocyte numbers. ATP arising from hemolysis was subtracted from the total ATP in supernatant to yield net ATP released by deoxygenation. Absolute numbers of ATP released from intact erythrocytes containing the murine deoxyHb-binding site (wild-type), the human deoxyHb-binding site (w/ human Hb site), and no deoxyHb site (w/o Hb site) in oxygenated vs deoxygenated condition are 44 ± 4.0 pmol/108 cells vs 67 ± 5.1 pmol/108 cells, 39 ± 3.1 pmol/108 cells vs 54 ± 1.4 pmol/108 cells, and 53 ± 1.5 pmol/108 cells vs 60 ± 3.0 pmol/108 cells.
Discussion
Although O2 regulation of RBC properties has been recognized for years,4,25,29,42,46-49 the mechanism by which this modulation is achieved has remained enigmatic. Well-characterized O2 regulatory pathways such as those involving HIF1α and von Hippel-Lindau protein do not exist in RBCs, and most other O2-binding proteins (eg, cytochromes) are also absent.50-52 The overwhelming abundance of Hb in erythrocytes has consequently been the focus of most hypotheses attempting to explain these phenomena.25,28,29 Although the reversible association of deoxyHb with band 3 has received the most attention,28-30,35 these speculations could not be tested in vivo for lack of an appropriate animal model. In this article, we show that the 3 most studied O2-dependent processes in RBCs rely on an intact deoxyHb-binding site on band 3. Our data demonstrate that residues 12 to 23 in human band 3 mediate the long-studied O2 regulation of RBC properties.
Crystal structures of oxyHb, deoxyHb, the cytoplasmic domain of band 3, and a complex between deoxyHb and the NH2-terminal peptide of band 3 provide useful information that can help explain the molecular basis of the above O2 molecular switch.32,33,53 These structures reveal that the NH2-terminal 55 residues of human band 3 are largely unstructured and that this highly flexible anionic segment can insert into the cationic central cavity of deoxyHb. Because this central 2,3-bisphosphoglycerate–binding cavity of deoxyHb narrows considerably upon O2 binding, oxyHb exhibits significantly reduced affinity for band 3; that is, creating a prominent O2-regulated protein-protein interaction in the red cell that switches on and off precisely over the range of O2 concentrations that are experienced by the erythrocyte in vivo.28
The proximity of the deoxyHb-binding site on band 3 to the docking sites of glycolytic enzymes on band 3 can explain the O2-mediated change in glucose flux between glycolysis and the pentose phosphate pathway.28,54 Thus, aldolase, GAPDH, and phosphofructokinase associate with sequences on band 3 that directly overlap the deoxyHb-binding site, and other GEs (eg, LDH and pyruvate kinase) interact at the COOH terminus of cdb3, which in the crystal structure also resides next to the deoxyHb-binding site.28,53-55 When deoxyHb binds to band 3, both GE-binding sites will be occluded, forcing displacement of the GEs from the membrane (Figure 3). Because the glycolytic enzymes are inhibited when bound to band 3,54 glucose flux is shifted toward the pentose phosphate pathway under oxygenated conditions. In contrast, when the cell is deoxygenated and deoxyHb displaces the GEs, glucose can be more aggressively consumed by glycolysis.22 Because RBCs require more nicotinamide adenine dinucleotide phosphate during periods of prolonged oxygenation to reduce the constantly formed methemoglobin back to its functionally normal Hb counterpart, we conclude that the O2-mediated reversible binding of GEs to band 3 is an important regulatory pathway in red cells.
The 2 ankyrin-binding loops on cdb3 reside in amino acids 63-73 and 175-185,56,57 which are located directly adjacent to each other and within ∼15 Å of the deoxyHb-binding site on cdb3. Although the crystal structure of intact ankyrin remains unsolved, one can calculate that the diameter of ankyrin would have to be at least 100 Å if it were a sphere.58 Given the known diameter of deoxyHb (∼55 Å), it would seem extremely unlikely for ankyrin to be able to occupy the same band 3 molecule as deoxyHb. We propose that deoxyHb will sterically displace ankyrin from band 3 whenever it occupies its binding site on band 3. Further, the probable adaptive advantage of this O2-mediated switch is that periods of deoxygenation should lead to rupture of multiple ankyrin-band 3 bridges, leading to increased deformability, allowing more rapid flow of deoxygenated RBCs from the tissues to the lungs.
ATP release through the pannexin-1 channel in RBCs is thought to be O2 dependent,59 suggesting that some type of communication between band 3 and pannexin-1 may exist. In addition, binding of deoxyHb to band 3 is known to induce global conformational changes in band 3 and associated membrane-spanning proteins.60-62 Taking these observations and our results together, we propose that the binding of deoxyHb to band 3 could trigger discharge of ATP through pannexin-1. The release of ATP into the vasculature upon RBC deoxygenation should promote generation of NO via activation of P2Y receptors on endothelial cells,46 thereby causing vasodilation and accelerated transit of the hypoxic RBCs to the lungs.
In summary, commonly held beliefs that the erythrocyte is an inert cell that passively circulates for 120 days before its removal by macrophages greatly underestimates the sensitive communication between this cell and its environment. We show here that multiple important RBC properties are exquisitely responsive to the oxygenation state of the cell, allowing the erythrocyte to better perform its gas transport function. Whether other O2-regulated RBC properties including NO release,4,63,64 Na+/K+ pumping,47,65 K+/Cl− cotransport,29,66 or Na+/K+/2Cl− cotransport29 are similarly regulated via the band 3–deoxyHb interaction will have to await further scrutiny.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health, National Institute of General Medical Sciences grant GM24417-36 (P.S.L.) and National Institutes of Health intramural funds (D.M.B., S.L.T.).
Authorship
Contribution: H.C., D.M.B., and P.S.L. designed the research, analyzed the data, and wrote the manuscript; and H.C., M.M.M., N.A.K., S.Z., L.M., S.L.T., and L.J.G. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip S. Low, 700 Clinic Dr, West Lafayette, IN 47907; e-mail: plow@purdue.edu; and David M. Bodine, Building 49, Room 4A04, 49 Convent Dr, MSC 4442, Bethesda, MD 20892; e-mail: tedyaz@mail.nih.gov.
References
Author notes
H.C., M.M.M., N.A.K., and S.Z. contributed equally.

![Figure 4. Effect of deoxygenation on band 3 retention in membrane cytoskeletons isolated from wild-type and transgenic mouse erythrocytes. (A) Erythrocytes from wild-type (left panel), transgenic mice containing a human deoxyHb-binding site (middle panel), or transgenic mice lacking a deoxyHb-binding site (right panel) were either oxygenated or deoxygenated prior to solubilization in 1% Triton X-100. Detergent-insoluble membrane fractions containing the spectrin/actin membrane skeletons were separated by SDS-PAGE and immunoblotted with anti-cdb3 or antiactin antibodies. (B) Densitometric analysis of the relative amount of band 3 retained in the above cytoskeletal pellets using actin as a loading control. All values of band 3 retention in cytoskeletons derived from deoxygenated RBCs are relative to the amount of band 3 retained in the pellet of the corresponding oxygenated erythrocytes (mean of 3 experiments ± standard deviation [SD]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/23/10.1182_blood-2016-01-692079/4/m_blood692079f4.jpeg?Expires=1769210538&Signature=ChzlE63Uo9tw8~dW~YLH9BUvbq4hCRHpt-qVCoWovy5Xl0PS9jFuIMf2JwntGWGimbA8BY8TTBWHRhSH6lqXNmf9IgIZ1yvYgkHWExD7gvudDrx9fx9t3OsL3na7rb8AS1RtjWeD43q5AGboB-atHQDfUZ1GmR1CwfGs6ZvR38eg0cgAAocyySMpwCx182lFiYo3m9cSvzRnvAtvzgVaXafK00Jvp2j0SuvNBmYSaIa2P-QGmYKGvAS4xkjbEoXbx7HEeXk15YdxwEmx1kmGnNk4nUtZH3ucglyf-gdTx1Q8CibFtO~hvsuMVPg1fdioEB58CLDY3s0MJIoIe6tgbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal