Abstract
The two Inhibitors of apoptosis proteins (IAPs), X-chromosome-liked linked IAP (XIAP) and cellular IAP1 (cIAP1), inhibit apoptosis and are important in leukemogenesis. Recent data suggest that both xIAP and cIAP play critical, non-overlapping roles in regulating innate and adaptive responses to certain stimuli. But the role of IAPs in allo-immunity is not known. We utilized distinct but complementary approaches, namely genetic and small molecule approaches to determine the role of IAPs in allo-immunity.
We first utilized AT-406, a small molecule IAP antagonist that is also known as second mitochondria-derived activator of caspase (SMAC) mimetic. This SMAC mimetic, is a pan-IAP inhibitor (inhibits both XIAP and cIAP) and reduces TNFα secretion in vitro. Because of the GVHD potentiating effects of TNFα, we hypothesized that treatment of allogeneic animals with AT-406 will reduce GVHD. We utilized B6→BALB/c MHC-mismatched BMT model. BALB/c recipients were lethally irradiated (8.5Gy) and transplanted withsyngeneic or allogeneic T cells along with bone marrow (BM). Both groups received either AT-406 or its diluent. Surprisingly, allo-recipients receiving AT-406 showed significantly worse GVHD severity and died more rapidly (p<0.05). This observation was also noted in another MHC disparate haploidentical B6→F1 model.
To further understand the role of IAPs, we next utilized genetic approach. When donor T cells from B6- cIAP-/- or XIAP-/- animals were compared to T cells from WT-B6, the allo-recipients (BALB/c) showed similar GVHD severity and mortality. Same results were also observed in a second B6→F1 model. Furthermore, in vitro studies showed that XIAP-/- and cIAP-/- T cells had comparable proliferation and cytokine secretion as WT-T cells. These data suggested that increase in GVHD mortality following treatment with AT-406 is not due to its effects on donor T cells.
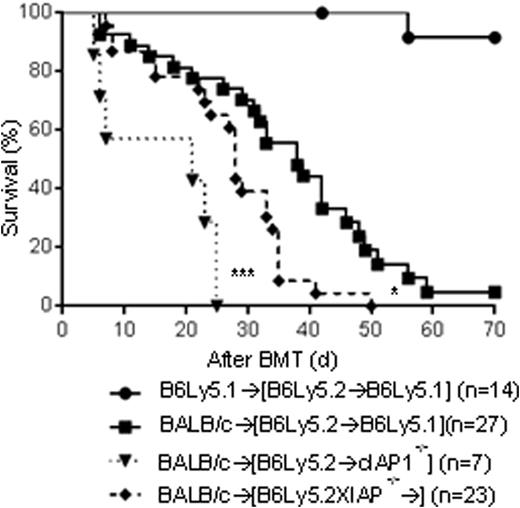
We therefore hypothesized that the absence of IAPs in hosts may impact on GVHD. To test this, cIAP-/-, XIAP-/- and WT-B6 animals were utilized as recipients in BALB/càB6 model. When compared with WT recipients both XIAP-/- and cIAP-/- recipients showed increased mortality (p<0.001) and worse clinical and histopathological GVHD (p<0.05, GI tract). We then determined whether IAPs expression on host hematopoietic derived cells is critical. We generated [B6→B6Ly5.2], [cIAP-/-→B6Ly5.2] and [XIAP-/-→B6Ly5.2] chimeras and utilized them as recipients in 2nd allo-BMT in BALB/càB6 models. All three chimeras, [B6→B6Ly5.2] [cIAP-/-→B6Ly5.2] and [XIAP-/-→B6Ly5.2] chimeras showed equivalent GVHD severity and mortality. Consistently, DCs from XIAP-/- and cIAP-/- animals showed similar functions as WT-B6 in vitro, suggesting that IAP expression in host hematopoietic-derived immune cells is not critical for GVHD. Next, to test the role of IAPs in non-hematopoietic GVHD target tissues, we made the reverse chimeras, namely, [B6Ly5.2→B6], [B6Ly5.2→XIAP-/-] and [B6Ly5.2→cIAP1-/-], where IAPs are absent only in the non-hematopoietic host cells. The allogeneic [B6Ly5.2→XIAP-/-] and [B6Ly5.2→cIAP1-/-] animals demonstrated a significantly worse survival compared to WT [B6Ly5.2→B6] recipient (p<0.01). To determine the potential mechanisms for enhanced mortality, we tested the expression of anti-apoptotic genes (Bcl-2, Bcl-xl) and autophagy (LC3) in the CD326+ intestinal epithelial cells from KO and WT animals harvested after allo-BMT. The cIAP-/- and -XIAP-/- animals showed significantly reduced expression of Bcl-2, Bcl-xl and LC3 than allo-WT animals. These data suggest that enhanced apoptosis and reduced autophagy in the target tissues in the absence of cIAP and XIAP caused greater GVHD. Thus expression of functional IAPs in host target tissues is crucial for reducing the damage from GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal