Abstract
Background: Mutational or non-mutational epigenetic events that aberrantly modify the chromatin regulatory machinery to enhance oncogene expression are a hallmark of myeloid malignancies. BRD4, a member of the bromodomain and extra terminal domain (BET) family, is a transcriptional coactivator that co-occupies super enhancer complexes associated with transcription of oncogenes (MYC, SOX2, NF-kB etc.) - and apoptosis regulators (Bcl-2, Bcl-XL, etc.) and has been validated as a target in AML therapy1.
ARV-825 is a hetero-bifunctional PROteolysis TArgeting Chimera (PROTAC) that recruits BRD4 to the E3 ubiquitin ligase cereblon and leads to efficient and sustained degradation of BRD4 resulting in down-regulation of MYC2.
Objectives: We examined the anti-leukemic effect of ARV-825 against AML cell lines, primary AML blasts and a mouse model of disseminated leukemia. Since tumor-stroma interactions driven by oncogene activation play a major role in resistance to AML therapy, we tested whether ARV-825 could overcome stroma-mediated drug resistance. As MYC harmonizes nutrient acquisition by cancer cells through regulation of the metabolites antiporter systems (SLC7A11, SLC5A5) 3, we profiled changes in a few important amino acids and organic acids in AML cell in response to ARV-825.
Results: The IC50s for all tested cell lines and primary AML cells at 72 hours were in the low nanomolar range (2-50 nM) and 10-100 times lower than JQ1, a small molecule BRD4 inhibitor. ARV-825 induces sustained BRD4 degradation accompanied by down-regulation of targets such as MYC, anti-apoptotic BCL-2 family molecules and an increase in apoptosis and DNA damage4.
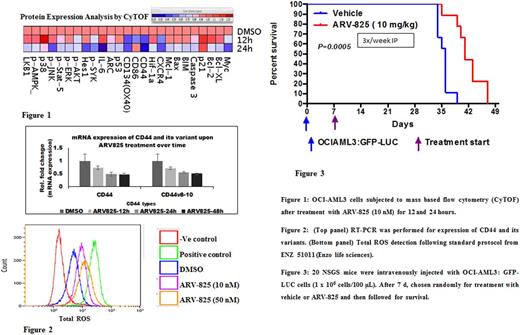
In an in vitro tumor-stroma co-culture model including hypoxic conditions, bone marrow derived mesenchymal stromal cells (MSCs) protected OCI-AML3 cells from cytarabine ( 55.4% apoptosis with vs. 35.0% without MSCs in normoxia and 50.6% vs. 32.8%, respectively, in hypoxia), but no such protection was observed against ARV-825 (58.7% apoptosis vs. 55.2% in normoxia and 57.4% vs. 58.3%, respectively, in hypoxia). Mass cytometry based proteomic analysis (CyTOF) (Fig. 1), immunoblotting and flow cytometry showed that among apoptotic, cell adhesion and signaling proteins, MYC, CD44 and surface CXCR4 were the most down-regulated proteins in AML cells. The functional relevance of surface CXCR4 down regulation was confirmed in migration assays against the CXCR4 ligand SDF-1. Phosphorylation of CXCR4 by PIM1 kinase is necessary for surface expression of CXCR4, ARV-825 treatment reduced PIM1 levels and phosphorylation of CXCR4 in AML cells while overexpression of PIM1 or Myc reversed the phenomena. Quantitative PCR and immunoblotting analysis confirmed the transcriptional down regulation of total CD44 and CD44 variants 8-10 (2-fold change treated vs. untreated). As a functional correlate of CD44 variants, mass spectrometry based intracellular metabolomics and flow cytometry confirmed reduction in cysteine uptake and increased reactive oxygen species (ROS) generation (Fig. 2). Additional metabolic readouts using the Sea horse system revealed inhibition of mitochondrial respiration as indicated by decreased in oxygen consumption rate and production of ATP upon treatment of ARV-825. Furthermore, array based gene expression profiling showed down-regulation of additional amino acid transporters and the Wnt/β-catenin pathway.
Finally, in a mouse model of human leukemia, the leukemia burdens were significantly lower in the ARV-825 treated mice as confirmed by luciferase imaging, flow cytometry, spleen size and survived longer compared to control mice (p=0.0005) (Fig.3).
Conclusion: ARV-825 has substantial, broad anti-AML activity and importantly modulates the tumor microenvironment and metabolism to overcome stroma-mediated drug resistance. Together, our data suggest that ARV-825 is an effective in targeting BET family of proteins for the treatment of AML
Reference:
1. Nature 2011; 478(7370): 524-528. doi: 10.1038/nature10334
2. Chem Biol 2015; 22(6): 755-763. doi: 10.1016/j.chembiol.2015.05.009
3. Cancer Res 2015; 75(9): 1782-1788. doi: 10.1158/0008-5472.CAN-14-3745
4. 604(675): ASH 2015,San franscisco,USA.
Lorenzi:Erytech Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: NIH-held patent related to L-asparaginase. Qian:Arvinas, LLC: Employment. Kantarjian:Bristol-Myers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal