Abstract
Integrins function as bi-directional signaling transducers that regulate cell-cell and cell-matrix signals across the membrane. A key modulator of integrin activation is talin, a large cytoskeletal protein that exists in an autoinhibited state in quiescent cells. Due to the fundamental importance of integrin activation in a myriad of critical cellular processes, including migration, adhesion, morphology, and proliferation, the mechanistic basis for molecular regulation of talin activation has been under intense investigation. A major mechanism of integrin activation in platelets is through heterotrimeric G protein signaling regulating hemostasis and thrombosis. Here we provide evidence that Switch Region 2 of the ubiquitously expressed G protein (Ga13) directly interacts with talin, relieves its state of autoinhibition, and triggers integrin activation. This Ga13-talin interaction represents a novel mechanism for the regulation of integrin signaling.
The most abundant platelet integrin complex, aIIbb3, is activated through the cytoskeletal protein talin. Talin is a large 235 kDa protein comprised of an N-terminal 45 kDa FERM domain, also known as the talin head domain (THD), and a series of helical bundles known as the rod domain. The talin head domain consists of 4 distinct lobes designated as F0-F3. Integrin binding and activation is mediated through the F3 region; a critically regulated domain in talin. Regulation of the F3 lobe is accomplished through autoinhibition via anti-parallel dimerization. In the anti-parallel dimerization model, the rod domain region of one talin molecule binds to the F3 lobe on an adjacent talin molecule, thus achieving the state of autoinhibition. Because platelet functionality requires integrins for adherence and thrombus formation, regulation of talin presents a critical node where pharmacological intervention is possible. Our experiments demonstrate that Ga13SR2 peptide recognized a single binding site on THD that is critical for talin autoinhibition. Subsequent experiments show a GST-SR2 fusion protein can sterically interfere with talin's autoinhibitory rod domain, and similar results were obtained using the full length Ga13. Furthermore, talin activity was investigated by intramolecular FRET. We established a novel assay using a full length talin molecule containing an N terminal GFP tag, and a red fluorescent dye binding motif incorporated into the autoinhibitory rod domain. In an autoinhibited anti-parallel dimer state, GFP acts as a donor to the red fluorescent dye on an adjacent talin molecule. The FRET signal would decrease when talin is activated. Using this assay, we show that Ga13 reduced FRET signal as compared to a Ga13 SR2 mutant (R227A) thus demonstrating Ga13 can activate talin in a cellular milieu.
To investigate the therapeutic potential of Ga13 SR2, we evaluated two critical aspects of anti-thrombotic therapy; bleeding time and in vivo thrombosis. Upon administration of Ga13SR2 peptide in mice, the bleeding time was significantly less than aspirin-treated mice. In vivo thrombosis was assessed using a laser injury model. A significant decrease in thrombosis was observed in mice treated with Ga13 SR2 when compared to the scramble peptide. Together, these studies suggest that the Ga13 SR2-talin activation pathway represents a novel integrin signaling process triggering rapid recruitment and activation of circulating platelets to the site of vascular injury. Because of the highly conserved nature of Ga13, talin, and integrins, the broad implications of this regulatory mechanism may extend to a multitude of cell types and pathologies.
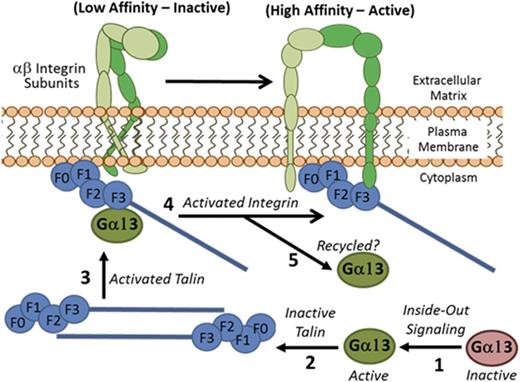
Ga13 mediated activation of talin by relieving anti-parallel dimerization. Inactive Ga13 (GDP bound) is activated by exchanging GDP for GTP, via intracellular signaling processes. Active Ga13 (GTP bound) relieves talin autoinhibition by displacing the head/rod domain interaction. Activated talin is free to bind the cytoplasmic tail of b integrins and facilitate integrin activation.
Ga13 mediated activation of talin by relieving anti-parallel dimerization. Inactive Ga13 (GDP bound) is activated by exchanging GDP for GTP, via intracellular signaling processes. Active Ga13 (GTP bound) relieves talin autoinhibition by displacing the head/rod domain interaction. Activated talin is free to bind the cytoplasmic tail of b integrins and facilitate integrin activation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal