Abstract
BACKGROUND: The study of rare diseases is limited by the uncommon nature of the conditions as well as the widely dispersed patient populations. Current rare disease registries such as the National Organization of Rare Diseases utilize centralized platforms for data collection; however because of their broad nature, these do not always capture unique, disease specific elements. Hairy Cell Leukemia (HCL) is a rare leukemia globally with approximately 900 new cases diagnosed in the US each year. The HCL Foundation undertook creation of a Patient Data Registry that collects data from multiple HCL Centers of Excellence (COE) around the globe to better understand the complications, treatment outcomes, disease subtypes, comorbid conditions, epidemiology, and quality of life of patients with HCL.
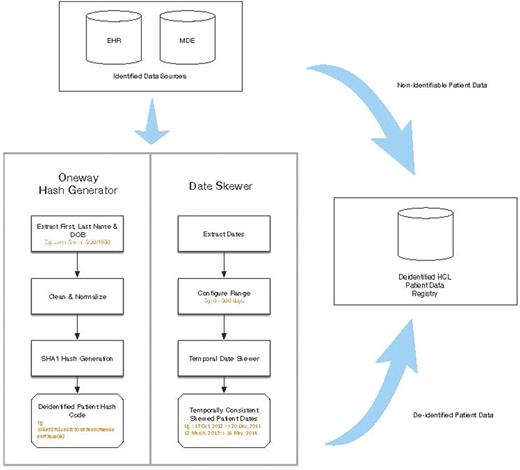
METHODS: Investigators at The Ohio State University Department of Biomedical Informatics and Division of Hematology in collaboration with the HCL Foundation developed a Patient Data Registry (PDR) for the longitudinal capture of high quality research data. This system differs from other registries in that it uses a federated( rather than centralized) architecture, wherein data is queried and integrated in an on-demand manner from local registry databases at each participating site. Further, the data collected for use in the registry combines both automated exports from existing electronic health records (EHRs) as well as additional data entered via a set of web-based forms. All manually entered data comes from source documents, and data provenance spanning electronic and manually entered data is maintained via multiple technical measures. Patients may be enrolled at HCL COE, or, if they do not have access to a COE they may enroll via a web-based portal (www.hairycellleukemia.org). At this time due to regulatory requirements the web-based portal is available to US patients only. All data are de-identified (see Figure 1: De-Identification Workflow) which reduces regulatory burden and increases opportunities for data access and re-use. End users have access to data via a project-specific query portal.
RESULTS: The Patient Data Registry has been deployed at The Ohio State University, Royal Marsden Hospital, and MD Anderson Cancer Center, and is undergoing deployment at the University of Rochester. Up to 25 international HCL COE may participate. In addition, US patients are actively entering the registry via the web-based portal. To date, 227 patients have been consented to the registry with 119 of these being via the web-based entry point.
CONCLUSION: We created an international and web-based patient data registry which will enable researchers to study outcomes in HCL in ways not previously possible given the rarity of the disease.
This work was made possible by research funding from the Hairy Cell Leukemia Foundation.
Andritsos:Hairy Cell Leukemia Foundation: Research Funding. Anghelina:Hairy Cell Leukemia Foundation: Research Funding. Lele:Hairy Cell Leukemia Foundation: Research Funding. Burger:Pharmacyclics: Research Funding. Delgado:Gilead: Consultancy, Honoraria; Novartis/GSK: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Infinity: Research Funding. Jones:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, LLC, an AbbVie Company: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lozanski:Beckman Coulter: Research Funding; Genentech: Research Funding; Stemline Therapeutics Inc.: Research Funding; Boehringer Ingelheim: Research Funding. Montserrat:Morphosys: Other: Expert Testimony; Vivia Biotech: Equity Ownership; Gilead: Consultancy, Other: Expert Testimony; Pharmacyclics: Consultancy; Janssen: Honoraria, Other: travel, accommodations, expenses. Parikh:Pharmacyclics: Honoraria, Research Funding. Park:Genentech/Roche: Research Funding; Amgen: Consultancy; Juno Therapeutics: Consultancy, Research Funding. Robak:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Tam:janssen: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Heckler:Hairy Cell Leukemia Foundation: Research Funding. Payne:Hairy Cell Leukemia Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal