Abstract
Background:PQR309 is an oral balanced, pan-PI3K, mTORC1 and mTORC2 inhibitor. It is in clinical development for the treatment of solid tumors and hematologic malignancies. 1st generation mTOR inhibitors inhibit the activity of mTOR within the TORC1 complex only with activation of TORC2 proposed as a putative resistance mechanism. PI3 kinase inhibition may reduce subsequent AKT activation which can bypass some effects of mTOR inhibition. Potent antiproliferative activity of PQR309 was previously demonstrated in lymphoma cell lines in vitro and in vivo. Maximum tolerated dose (MTD) of PQR309 in solid tumours was established at 80 mg using a continuous once daily dosing schedule (OD).
Methods:We performeda modified 3+3 DE of PQR309, open label phase 1 trial with expansion, to evaluate safety, pharmacokinetics (PK) and efficacy. Patients with relapsed or refractory lymphoma (any sub-type, ECOG PS of 0-1) were treated in two sequential cohorts with escalating doses of PQR309 administered on an OD dosing schedule to assess the MTD of PQR309. The starting dose of PQR309 was 60mg OD. The dose limiting toxicity (DLT) period was the first cycle of treatment, 21 days (d). PK samples were obtained at predefined time points. Clinical efficacy was evaluated according to revised Cheson criteria. In the expansion phase, patients will be treated at the MTD as defined in the DE phase of the study.
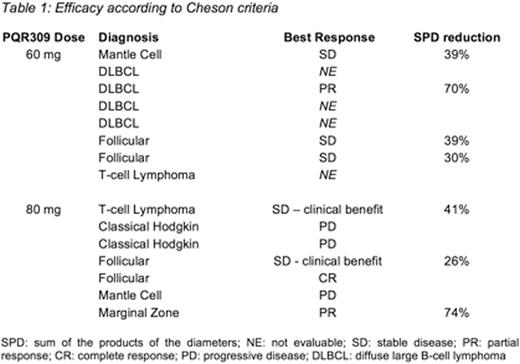
Results: 15 patients were enrolled between August 2015 and March 2016 and treated with 60mg (n=8) or 80mg (n=7) of PQR309. Demographics: 5F:10M; median age 60 (range: 34-75), median number of prior systemic treatments 5 (range: 1-8). Lymphoma indications are shown in Table 1. Mean duration on therapy was 39 days (range: 3-160). One patient with follicular lymphoma remains on treatment. Grade (G)3/4 drug-related AE were seen in 3 patients treated with 60mg: 1 G4 rhabdomyolysis, 1 G4 neutropenia, 1 G3 hyperglycemia and one patient who developed G3 anorexia and G4 sepsis. Four patients treated with 80mg developed G3/4 drug-related AEs: two patients developed G3 hyperglycemia, one patient developed G3 fatigue and G3 pneumonitis. No DLT was observed. Preliminary PK showed rapid absorption (Tmax 1-2h), dose proportionality for Cmax and AUC and an estimated T1/2 of around 50 hours, consistent with PQR309 studies in solid tumours that evaluated dose levels from 10 to 150 mg PQR309.
Responses observed in each patient are shown in the table below. 4 patients were non-evaluable, 3 due to disease progression requiring cessation of study drug and one requiring steroid doses exceeding protocol defined criteria, all within the 21 day DLT assessment period.
Conclusion:The MTD and recommended PQR309 dose for the expansion of the study was 80mg OD, in agreement with earlier dose-finding studies in solid tumours. Adverse event patterns were consistent with those seen in studies involing solid tumours. Hyperglycemia, a predicted on-target effect of PI3K/mTOR inhibition, was observed in the majority of patients, providing evidence of pharmacodynamic effects of PQR309. PK was dose-proportional. Encouraging clinical activity including a CR was observed. The study expansion is ongoing.
Collins:Takeda: Consultancy, Honoraria, Speakers Bureau. Eyre:GSK: Honoraria; Celgene: Other: Travel, Accomodation; Gilead: Honoraria, Other: Travel, Accomodation, Speakers Bureau; Takeda: Honoraria, Other: Travel, Speakers Bureau. Ivanova:PIQUR: Employment. Schmitz:PIQUR: Employment. Dimitrijevic:PIQUR: Employment. Dreyling:Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal