Abstract
Current practice in the prevention of venous thromboembolism (VTE) is either controlled dosage of vitamin K antagonists (VKA) or standard dosage of a low molecular weight heparin (LMWH) or an orally administrated direct inhibitor of thrombin or factor Xa (DOACs). This is justified by clinical trials showing the non-inferiority of standard dosage to VKA treatment - disregarding that the latter could be improved upon. Whether standard dosage is optimal for the individual patient depends upon how well (s)he is represented by the "average" patient that meets the inclusion criteria of the trial. Here we show that in a set of normal plasmas the individual thrombin generating power (the endogenous thrombin potential: ETP), after spiking with a fixed concentration (~ IC50) of different anticoagulants show such a wide variation that, even when plasma levels would be identical, a considerable percentage of patients could be over- or under-anticoagulated. Consequently adapted doses must be considered if better results than those with VKA are our aim.
We recall that the ETP is close to constant in the individual person but varies in the population with a broad log-normal distribution (CV 16%). The ETP is highly correlated to the risk of thrombosis: The relative risk of VTE in the upper quartile being 5 - 7 times higher than that in the lower one (Winckers K., et al., poster PO617, ISTH 2015, Toronto).
Reducing the ETP is the common feature of all antithrombotic therapy. The desired range of reduction is not exactly known. In congenital bleeding disorders bleeding risks increase sharply at ETP below 33%. In monitoring VKA treatment an INR below 2, which corresponds to an ETP > 66% of normal, is generally considered inadequate. We therefore arbitrarily choose the range 33 - 66% of normal, as a target range (TR).
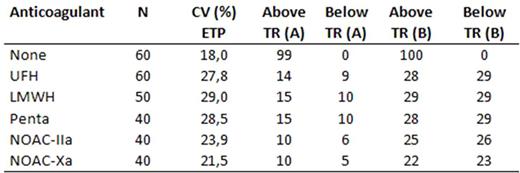
Experimental: Individual plasmas from 60 normal healthy volunteers were spiked with a fixed amount of either unfractionated heparin (UFH), low molecular weight heparin (LMWH), antithrombin binding pentasaccharide (penta), DOAC acting on thrombin or DOAC acting on factor Xa. We found the residual ETP to be highly variable (table column 3), obviously because the variation in susceptibility to the inhibitor superimposes upon the natural variation. Using these CVs we calculated what % falls either above or below the target range.
The total variance of the ETP under treatment is the combined effect of natural variance, the pharmacokinetic- and the pharmacodynamic -variance. Here we determined pharmacodynamic effects only and calculated the % of ETP values that would fall outside the target range A: if there would be no pharmacokinetic variance (table column 4 & 5) and B: If the pharmacokinetic variance would equal the pharmacodynamic variance (table column 6 & 7).
It is seen that in any case over 15% of the population will be outside the target range and that in the more likely case that pharmacokinetic variation counts as much as pharmacodynamic variation does around half of all patients will be outside the safe range. This could be avoided by measuring the effect of a standard dose of anticoagulant on the ETP once and increasing the dose in those with an ETP > 66% and decreasing it in those with ETP < 33%.
The present work is meant to provide the rationale for starting clinical studies on the actual variation of the ETP attained under standard dosage of different anticoagulants and the effects thereon of personalised dose adjustment.
Hemker:Diagnostica Stago: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal