Abstract
Introduction:Even in the era of novel targeted therapies for the treatment of Chronic Lymphocytic Leukemia (CLL) patients, such as BTK, PI3K and BCL2 inhibitors, allogeneic hematopoietic stem cell transplantations (alloHCT) will remain an important treatment option for a subset of patients with very high risk CLL. The current study focused on the impact of center and procedure-related factors on outcomes after alloHCT, taking into account the impact of patient- and disease-related risk factors.
Patients and Methods:Data of 684 CLL patients who received a first alloHCT between 2000 and 2011 were analyzed. Their data were collected as part of the EBMT CLL Data Quality Initiative. Outcomes of interest were Event-Free Survival (EFS) up to 5 years after transplantation and mortality in the first 100 days after alloHCT. Outcomes were analyzed by means of the Kaplan-Meier method and Cox proportional hazards models with a frailty (random effects) component to take into account unexplained center heterogeneity. The following factors describing center characteristics or the transplant procedure were analyzed: experience in alloHCT in general and, for CLL specifically, accreditation by the Joint Accreditation Committee-ISCT & EBMT (JACIE), Gross National Income (GNI)/capita based on purchasing power parity (PPP) (GNI/cap), donor type, donor-patient sex-match, type of conditioning, stem cell source and T-cell depletion (TCD).
Results:Five-year EFS of the whole cohort was 37% (95% Confidence Interval, 33%-42%), Day-100 survival was 90% (88%-92%). Experience of the transplant center was measured by the number of all alloHCTs, and alloHCTs for patients with CLL respectively. The median total number of alloHCTs per center per year was 45 (range 0-169) and the median number of CLL alloHCTs was only 2 per center per year (range 0-19). Greater experience with transplantation of patients with CLL (Hazard Ratio (HR) 0.96 per additional transplant, p=0.002), JACIE accreditation (HR 0.7, p=0.045) and a higher GNI/cap (HR 0.4, 95% CI 0.2-0.96, p=0.04) showed a protective impact on 5-year EFS in the Cox model. In vivo TCD with alemtuzumab (HR 1.5 compared to no TCD, p=0.03) and a female donor for a male patient (HR 1.4 compared to a male donor for a male patient, p=0.02) were the only procedure-related factors significantly associated with EFS. Event-Free Survival after in vivo TCD with Anti-Thymocyte-Globulin or after ex vivo TCD was comparable to EFS without TCD (HR 0.9, 0.7-1.3, p=0.6; HR 0.9, 0.5-1.6, p=0.8). Non-myeloablative conditioning did not have a negative impact on 5-year EFS, and exposed patients to a lower risk of non-relapse mortality. Measured and unmeasured center characteristics did not have a significant impact on 100-day mortality.
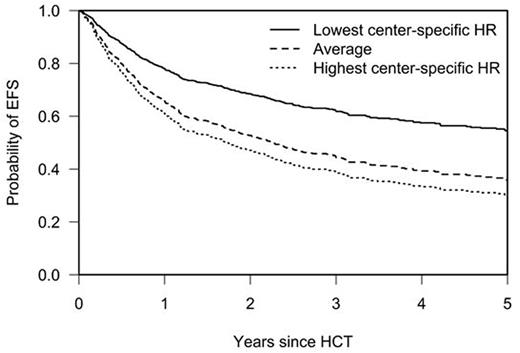
Even when correcting for patient-, procedure- and center-related characteristics, there was still significant variation in center outcome, expressed by center-specific HRs derived from the frailty models, ranging from 0.6 to 1.2. Their impact is illustrated in a model-based plot for EFS (see Figure) which shows outcomes for three reference patients with the same characteristics who would be transplanted in three centers with the same measured characteristics but with the highest, average and lowest HRs in the dataset. These unexplained center effects likely represent a mixture of differences which could apply to the location of the transplant center, unmeasured characteristics of the patient population transplanted at this center, selection criteria which were not reported and factors determining the success of the transplant procedure which might differ between centers.
Conclusion: We have confirmed that both center- and procedure-related factors have a significant impact on the EFS of patients with CLL undergoing alloHCT. Our results may help to interpret outcomes of single or multicenter studies better. Since non-myeloablative conditioning did not have a negative impact on EFS and exposed patients to a lower risk of non-relapse mortality, this approach should be favored for future alloHCT for CLL.
Probability of Event-Free Survival up to Five Years Post-HCT for three Reference Patients
Contribution: J.S. designed the research and wrote the paper. L.C.d.W conducted the statistical analysis and produced the figure.
Schetelig:Sanofi: Honoraria. Gramatzki:Janssen: Other: Travel/Accommodation/Expenses, Research Funding. Dreger:Gilead: Consultancy; Gilead: Speakers Bureau; Janssen: Consultancy; Novartis: Speakers Bureau; Novartis: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal