Abstract
Introduction: Cell source and graft composition are known to have a prognostic role in allogeneic hematopoietic cell transplant (HCT). Currently, there are no data in the setting of haploidentical-HCT with post-transplant cyclophosphamide (PT-Cy).
Patients and methods: One-hundred patients undergoing haplo-HCT with PT-Cy from Sept 2011 to Nov 2015 were included in this multicenter retrospective analysis.
Bone marrow (BM) was used as graft source in 25 patients and peripheral blood stem cell (PBSC) in 75. The two groups (BM vs PBSC) were similar in terms of age, Disease Risk Index (DRI) and HCT-CI. The only differences were on disease type (lymphoid malignancies 76% vs 57%, p=0.02) and conditioning regimen (myeloablative: 100% versus 30%, p<0.01).
Graft cellular subsets analysis was performed by means of flow cytometry (CD34, CD3, CD4, CD8, CD56, CD19, CD38, CD31, CD25, CD45RA, CD95, CD127, CCR4, CCR6, CCR7). Overall Survival (OS) and Progression Free Survival (PFS) were performed with Kaplan-Meier analysis. Acute GVHD, chronic GVHD, Non-Relapse Mortality (NRM) and Relapse Incidence/Progression of disease (RI/POD) were obtained with competing risk analysis.
Results: For the whole cohort, neutrophil and platelet engraftment cumulative incidences at day +30 were 97% (95% CI: 91-99) and 64% (95% CI: 53-73), with no differences between BM or PBSC. Grade II-IV and grade III-IV aGVHD cumulative incidences at day +100 were 36% (95% CI: 26-48) and 10% (95%CI: 5-18), respectively. Acute GVHD cumulative incidence was significantly lower for BM grafts [16% (95% CI: 5-33) vs 43% (95% CI: 31-54), p=0.02], but no differences were observed regarding grade III-IV acute GVHD [8% (CI 95%: 1-24) vs 11% (CI 95%: 5-20), p=0.73]. Chronic GVHD cumulative incidence at 18 months was 23% (95%CI: 15-32) with no difference between BM or PBSC. With a median follow up of 16.5 months (range 0.8 - 40.6), the 18-months PFS and OS were 43% (95%CI: 32-54) and 63% (95% CI: 52-73), respectively. NRM and RI/POD at 18 months were 18% (95%CI: 11-26) and 38% (95%CI: 27-49).
Multivariable analysis was performed using patient (age, gender, HCT-CI, DRI) and transplant characteristics (donor gender, donor-recipient relation, graft source). A DRI>1 was the only factor associated with higher RI/POD (HR 3.45, 95% CI: 1.4-8.2, p<0.01), lower PFS (HR 2.77, 95%CI: 1.4-5.3, p<0.01), and lower OS (HR 2.33, 95%CI: 1.1-4.9, p=0.02). Graft source did not influence survival outcomes.
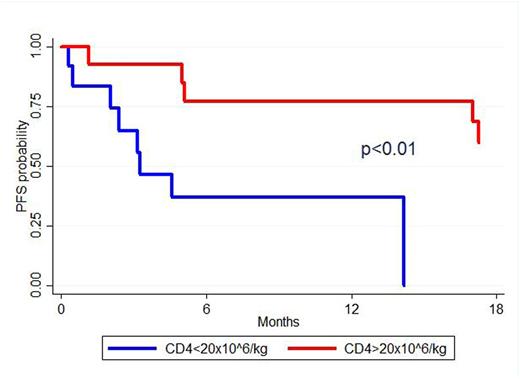
Graft cell composition analysis was performed separately for BM and PBSC cohorts. For the BM group, univariable analysis showed that CD4 graft count ≥20x10^6/kg was associated with a prolonged PFS [60% (95%CI: 28-81) vs 0%, p<0.01] (fig. 1), a trend toward a decreased NRM [0% vs 27% (95%CI: 5-56), p=0.05] and a prolonged OS [92%(95%CI: 57-99) vs 58% (95%CI:27-80), p=0.05]. Among the CD4 subsets analyzed (activated, central memory, effector, effector memory, memory stem cells, regulatory, Th17), naive T cells ≥8.5x10^6/kg (CD3+/CD8-/CD4+/CCR7+/CD45RA+/CD95-) and recent thymic emigrants ≥6.9x10^6/kg (median) (RTEs, CD4+/CD31+/CD45RA+) were the only phenotypes associated with a better PFS and OS (p=0.02 and p=0.04 respectively). For the PBSC group, CD8 graft count ≥ 75 x10^6/kg (median) was associated with a lower NRM [3% (95%CI: 0-12) vs 16% (95%CI:6-30), p=0.01]. No associations were found regarding graft cell composition and incidence of acute or chronic GVHD for both BM and PBSC groups.
Conclusions: In our retrospective analysis, we did not observe significant differences in survival outcomes based on graft source. Patients receiving BM grafts developed fewer grade II-IV aGVHD, but grade III-IV aGVHD incidence was similar between the two groups.
For the BM group, a higher CD4 count was predictive of better PFS, TRM and OS, as reported with standard GVHD prophylaxis and matched donors (Waller EK, J Clin Oncol 2014. 32: 2365-2372). Interestingly, of the CD4 population, only naive T cells and RTEs were associated with an improved PFS and OS. A protective effect of CD8 cell count was observed in the PBSC group. This is similar to the non PT-Cy setting where higher CD8 cells are associated with better survival outcomes (Reshef R, J Clin Oncol 2015). These data should be validated in a prospective analysis to guide the choice of cell source in the haplo PT-Cy setting.
Kaplan-Meier estimates of progression free survival from time of transplant associated to the CD4 graft content.
Kaplan-Meier estimates of progression free survival from time of transplant associated to the CD4 graft content.
Corradini:Celgene: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Roche: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Servier: Honoraria; Gilead: Honoraria; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal