Abstract
Background: TET2, located on chromosome 4q24, is frequently mutated in the majority of patients with chronic myelomonocytic leukemia (CMML). TET2 has 11 exons, and variations, especially in exon 3 have been described as a part of age related clonal hematopoiesis (Jaiswal NEJM 2015). In CMML, somatic TET2 mutations in the absence of ASXL1 mutations (ASXL1wt/TET2mt) were previously shown to impart a favorable outcome on survival (Patnaik BCJ 2015). In the current larger CMML patient cohort, we describe the number and type of TET2 mutations and examine their phenotypic and prognostic effects.
Methods: 261 patients with CMML were included in the study. All patients had bone marrow (BM) biopsies and cytogenetics performed at diagnosis. Targeted capture assays were carried out on BM DNA specimens obtained at diagnosis for the following genes; TET2, DNMT3A, IDH1, IDH2, ASXL1, EZH2, SUZ12, SRSF2, SF3B1, ZRSR2, U2AF1, PTPN11, Tp53, SH2B3, RUNX1, CBL, NRAS, KRAS, JAK2, CSF3R, FLT3, KIT, CALR, MPL, NPM1, CEBPA, IKZF, and SETBP1. TET2 (NM_001127208.2) coverage extended from exons 3-11, with frame shift, non-sense, and missense variations considered pathogenic. Previously annotated single nucleotide polymorphisms (http//www.hapmap.org) were considered non-pathogenic.
Results:Among the 261 study patients, 65% were males and median age was 70 years (range, 28-91). 154 (59%), 64 (25%) and 43 (16%) patients were classified as CMML-0, 1 and 2, respectively. At a median follow-up of 23 months, 174 (67%) deaths and 37 (14%) leukemic transformations were documented. Mutational frequencies ≥4% were encountered in; ASXL1 45%, TET2 42%, SRSF2 40%, NRAS 14%, SETBP1 13%, CBL 10%, JAK2 7%, RUNX1 6%, DNMT3A 6%, U2AF1 6%, SF3B1 5%, ZRSR2 4%, Tp53 4% and IDH2 4%.
i) TET2 mutations type, number and phenotypic correlates:
TET2 mutations were seen in 109 (42%) patients; these included frameshift 33 (30%), nonsense 29 (27%) and missense 11 (10%) variants whereas 36 (33%) had more than one type of mutation. Overall, 57 (52%) patients had more than 1 TET2 mutations: 52 (48%) patients had 1, 46 (42%) 2 and 11 (10%) ≥3 TET2 mutations. Among all 109 TET2 mutated patients, 65% were male, and median age was 71 years with no significant difference in age and gender distribution between mutated and un-mutated cases, or type of TET2 mutations; however, older patients were more likely to carry multiple TET2 mutations (p=0.01). Compared to their un-mutated counterparts, TET2 mutated cases were less likely to have a low hemoglobin (p<0.001), circulating immature myeloid cells (IMC) (p=0.001), peripheral blood (PB) (p=0.009) and BM blasts (p=0.009), and have high-risk stratification per Mayo Molecular Model (p<0.001); these differences were not affected by the type or number of TET2 mutations. TET2 mutated cases were more likely to have a higher frequency of SRSF2 (p=0.004) and lower frequency of ASXL1 (p=0.03), Tp53 (p=0.04) and IDH1/2 mutations (p<0.001); these associations were also not affected by the type or number of TET2 mutations.
ii) Impact on survival:
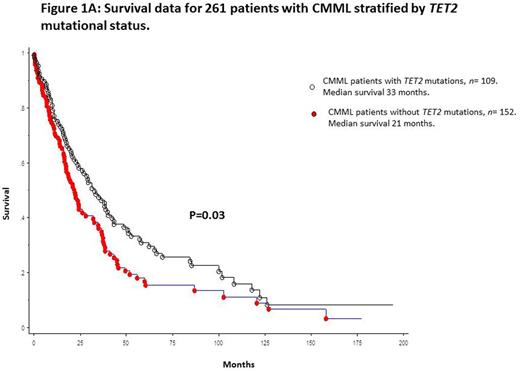
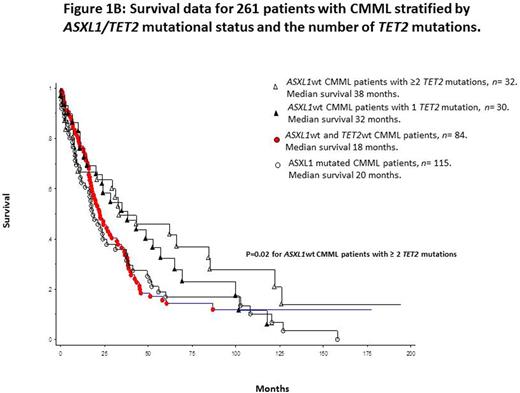
Median survival for the entire cohort (n=261) was 24 months. In univariate analysis, survival was superior in TET2 mutated (median 33 months) versus wild-type (median 21 months) patients [p=0.03; HR 1.3 95% CI 1.12-1.86] (figure 1A). This survival difference remained significant after adjustment for age (p=0.04), leukocyte count (p=0.017), absolute monocyte count (p=0.02), absolute lymphocyte count (p=0.02), platelet count (p=0.015), circulating IMC (p=0.03), DNMT3A (p=0.02) and ASXL1 (p=0.045) mutations; however, significance was lost after adjustment for abnormal karyotype (p=0.32) and the Mayo Molecular Model (p=0.0003). These observations were not affected by the type or number of TET2 mutations. Finally, our previous observation regarding the survival advantage of ASXL1wt/TET2mt versus other genotypes was most apparent for patients with multiple TET2 mutations (p=0.02) (figure 1B).
Conclusions:TET2 mutations are common in CMML and constitute approximately equal proportions of frameshift and non-sense mutations, while missense mutations are less frequent. Majority of TET2 mutated CMML cases harbor more than one mutant variant. Regardless, the relevance of type and number of TET2 mutations in CMML was limited to an association between older age and number of mutations and the latter with possibly improved survival in the absence of clonal ASXL1 mutations.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal