Abstract
Introduction: The outcomes of patients (pts) with post-MPN AML are poor, and there is no standard treatment. The Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib (RUX) markedly improves symptoms and splenomegaly and extends overall survival in advanced myelofibrosis (MF). In a phase 2 study of RUX in pts with relapsed/refractory (R/R) leukemias, 3 of 18 pts with post-MPN AML achieved a complete response (CR) with or without sufficient count recovery and RUX was very well tolerated up to 50 mg twice daily (bid, Eghtedar et al. Blood 2012). The hypomethylating agent (HMA) decitabine (DAC) is widely used for the treatment of AML. HMAs have significant activity in post-MPN AML (Thepot et al. Blood 2010, Mascarenhas et al. Leuk Res 2010, Badar et al. Leuk Res 2015). The combination of RUX and DAC provides an opportunity to target post-MPN AML through disparate mechanisms.
Methods: All pts with R/R AML were eligible in the phase I portion of this ongoing, phase I/II trial. Pts in the phase II portion must have post-MPN AML. DAC is administered at 20 mg/m2/d x 5 days in each cycle and RUX is administered continuously starting day 1 of DAC. Cycle length is 4-6 weeks. In the phase I portion, the RUX dose was escalated in "3+3" fashion from 10 through 50 mg bid (dose levels 0, 1, 2 and 3 = 10, 15, 25 and 50 mg bid). Dose limiting toxicities (DLTs) were defined in the first cycle. Response assessment is performed according to published criteria for AML (Cheson et al. JCO 2003). Pts may receive up to 24 cycles of therapy and may proceed to allogeneic stem cell transplantation (ASCT) at any time after cycle 1. Measurement of circulating cytokine levels, intracellular signaling proteins and gene methylation assays are to be performed after 1 and 3 cycles and every 3 cycles thereafter.
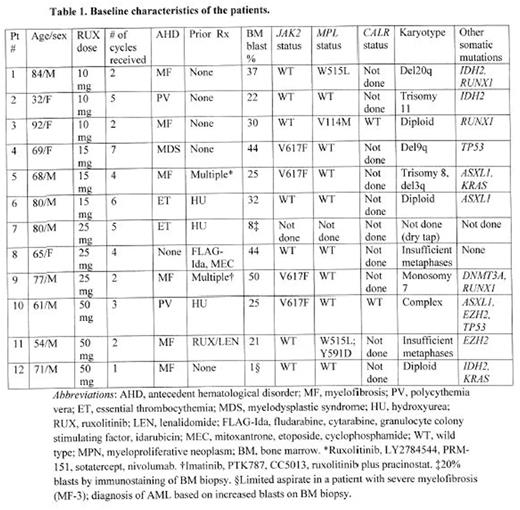
Results: The phase I portion of the study has completed accrual. 14 pts with AML were enrolled, of whom 2 did not complete 1 cycle of treatment and hence were not evaluable for DLT (withdrew consent; not considered further). The median age was 70 (range 32-92) and the median bone marrow blast percentage was 27.5 (range 1-50). All but 2 pts had post-MPN AML. Of these 2 pts, one (with AML arising from myelodysplastic syndrome (MDS)) had a JAK2 V617F mutation. Of the 10 pts with post-MPN AML, 5 had received no prior therapy for their MPN, 3 hydroxyurea (HU) alone, and 2 multiple treatments for MF. The pt with prior MDS was previously untreated, and the pt with AML without an antecedent hematological disorder had received 2 prior cytotoxic chemotherapy regimens (Table 1).
3 pts each received 10, 15, 25 and 50 mg of RUX bid. Most adverse events (AEs) were grades 1 or 2. Grade 3 or 4 AEs included pneumonia, febrile neutropenia, thrombocytopenia, neutropenia, infection, hypotension, fracture, fever, fatigue, anorexia, muscle weakness, confusion, ileus, and transaminitis; of these, only grade 3/4 thrombocytopenia and neutropenia were judged possibly related to the therapy. No DLTs occurred and the recommended phase 2 dose (RP2D) is RUX 50 mg bid with standard dose DAC. 4 of the 12 evaluable pts have died, 2 from pneumonia, 1 from sepsis and 1 from gastric hemorrhage, all considered unrelated to study treatment. 4 pts remain on study; 4 others discontinued due to disease progression. The median number of cycles received is 4 (range 1-7).
1 pt achieved a CR and 1 CR with incomplete platelet recovery (CRp) after 1 and 3 cycles, respectively; both remain on study treatment. These pts (pt 11 and pt 6 in table 1) received RUX at 50 mg bid and 15 mg bid, respectively. Another pt in the RUX 50 mg bid cohort (pt 10 in table 1) with AML with a complex karyotype and mutated TP53 achieved a CR with incomplete count recovery (CRi) after 1 cycle but subsequently relapsed. A fourth pt (pt 2 in table 1) obtained a partial remission after 2 cycles and proceeded to ASCT after 5. The last pt in the RUX 50 mg bid cohort (pt 12 in table 1) has received 1 cycle of treatment and achieved a morphologic leukemia-free state. The overall response rate is 42%.
Conclusion: The combination of RUX and DAC is tolerable and shows promising efficacy in post-MPN AML. Up to 24 pts with post-MPN AML will be enrolled in the phase II portion of the study, at the RP2D of 50 mg bid of RUX and 20 mg/m2/d of DAC x 5 days.
Jain:Celgene: Research Funding; Infinity: Research Funding; Seattle Genetics: Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Novartis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Abbvie: Research Funding; Novimmune: Consultancy, Honoraria. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. DiNardo:Celgene: Research Funding; Novartis: Research Funding; Agios: Research Funding; Abbvie: Research Funding; Daiichi Sankyo: Research Funding. Pemmaraju:Incyte Corporation: Consultancy, Honoraria. Kantarjian:ARIAD: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal