Abstract
PV is characterized by the gain-of-function V617F mutation in JAK2, the gene encoding the first signaling element of the cytokine receptor superfamily. Progenitor cells from PV are more sensitive in vitro to Imatinib, which also inhibits the SCF receptor cKIT, than those from normal sources (Gaikwad, Exp Hemat 2007;35:931) and clinical trials with similar tyrosine kinase inhibitors have been reported to have some efficacy in PV (Nussenzveig, Int J Hematol 2009;90:58; Silver, Leuk Res 2012;36:156). These data led us to examine the mechanism by which this tyrosine kinase inhibitor affects erythropoiesis in PV.
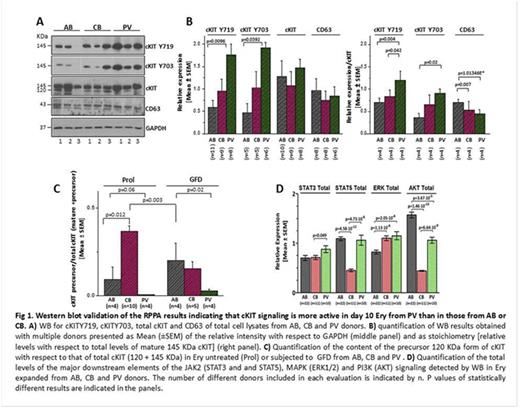
We observed that in cultures containing SCF PV progenitor cells generated similar numbers of erythroid cells (Ery) as those from adult (AB) and cord (CB) blood by day 10 [fold increase (FI) ~1-2] but by day 13 PV cells generated significantly greater numbers of Ery than AB and CB [PV FI=11±0.2, p=0.021 vs AB; CB FI=6.2±1.9, p=0.025 vs PV and 0.0055 vs AB; AB FI=2.6±0.5]. Since by day 10 progenitor cells were no longer detected, we hypothesized that increases in Ery at day 13 reflect intrinsically greater Ery proliferation potentials. To test this hypothesis, we compared the phosphoproteomic landscaping of day 10 Ery from 3 PV, 3 AB and 3 CB by Reverse Phase Protein Array (RPPA) using as target >160 signaling events (data are at http://capmm.gmu.edu/data). Overall, 40 proteins were statistically different between PV and AB and 30 proteins were statistically different between CB and AB. Pathway analyses of significant hits identified that PV and CB Ery differ from the AB ones in the activation states of 1-2 proteins involved in stemness and cell cycle control inferring that there is no major change in their cycling or differentiation state. By contrast, the 3 populations showed numerous differences in cKIT signaling. PV Ery differed from AB cells by expressing greater levels of cKITY719 and cKIT703, which were reduced to barely detectable levels by the pan-JAK inhibitor Ruxolitinib, and of elements of PI3K (eNOS/NosIII, PDK1 and PKCd) and MAPK (pMARCKS, MSK1, AMPKα1 and β1 and p38 MAPK) signaling downstream, respectively, to cKITY719 and cKIT703. PV Ery expressed also greater levels of JAK2Y1007/1008 and of its downstream target STAT3Y705. CB Ery showed lower levels of cKIT, cKITY703, cKITY719 and CD63, a member of tetraspanin superfamily that binds cKITY719 switching its intracellular fate from recycling to lysosome degradation, greater phosphorylation of proteins of MAPK (pMARCKS, MSK1, PTEN and Src) and PI3K (PKCd, mTOR, p70S6K and panPKC/βII) signaling than AB Ery. These results were stoichiometrically validated by WB and indicate that PV Ery express greater degrees of cKIT activation than AB Ery suggesting that greater response to SCF might account for their greater amplification in culture. This hypothesis was tested by RPPA analyses (and stoichiometric validation by WB) of Ery from PV, AB and CB growth factor deprived (GFD) for 4h and then stimulated with SCF for 15' and 2h. GFD altered the activation state of 25 proteins (22 de-activated and 3 activated) in PV, of 12 proteins (10 de-activated and 2 activated) in AB and 8 proteins (4 de-activated and 4 activated) in CB. SCF altered the activation state of 36 proteins in PV (18 activated and 18 de-activated), 23 proteins in CB (all activations) and 6 proteins in AB (all activations). In PV and CB Ery, GFD decreased cKITY719 and cKITY703 and the activation state of their downstream targets JAK2Y1007/1008, MAPKs and mTOR while SCF increased the stoichiometric levels of cKITY719 and cKITY703 and the activation of mTOR. SCF also increased cKITY703 and cKITY719 but did not activate mTOR in Ery from AB. In agreement with the hypothesis that Ery from PV and CB respond more readily to SCF than those from AB, SCF induced greater cell-surface cKIT down-modulation (by flow cytometry) and lower intra-cytoplasmic cKIT/CD63 association (by confocal microscopy and WB) in PV and CB Ery than in AB Ery. Screening of 97 inhibitors against targets analysed by RPPA which are approved for clinical use by FDA revealed that growth of PV Ery was more sensitive than that of AB only to JAK and cKIT inhibitors. In addition, shRNA-CD63 reduced the growth of PV Ery (FI=0.9 vs 1.3 p=0.012) while increased by 2-fold (p=0.02) that of AB Ery. These results provide the first phosphoproteomic landscaping of cKIT signalling in Ery from PV and normal sources and confirm that cKIT is an important therapeutic target for PV.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal