Abstract
Introduction: Phase I clinical trials are primarily designed to assess the safety and toxicity of a new agent and determine the recommended dose. Such trials are challenging as patients typically have multiply relapsed/ refractory disease and have received all standard therapies. Many have a poor prognosis and would otherwise receive supportive/ palliative treatment. However, the advent of molecularly targeted therapies and immunotherapies has challenged this paradigm. The outcomes of phase 1 trials in solid tumours have been reported, but not in haematological malignancies. Here we report the phase I/II trials experience from a dedicated trials unit within a large UK Haematology centre.
Methods: This was a retrospective review of patients with haematological malignancies sequentially treated in Phase I/II clinical trials at the NIHR/ UCLH Clinical Research Facility, London, UK. Patients met the relevant eligibility criteria for each study and received at least one dose of study medication. Adverse events were graded by CTCAE 4.0, response assessments as per trial protocol. Univariate and multivariate analysis was performed upon demographic, clinical and haematological/ biochemical parameters to investigate predictive markers of outcome. Kaplan Meier method was used for survival analysis and censored for those proceeding to ASCT.
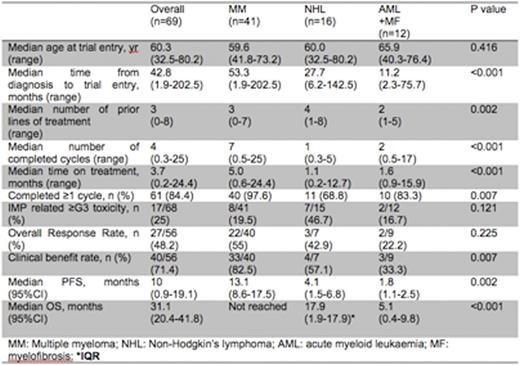
Results: 69 patients were enrolled onto 11 trials between March 2012 and July 2016. 6 were phase I studies (four first-in-human); 4 phase I/II studies; and one phase II study with a dose finding phase. All involved molecularly or immunologically targeted therapies. Median age was 60.3 yrs (range 32.5-80.2). The median time from diagnosis to trial treatment was 42.8 months (range 1.9-202.5). Disease types included multiple myeloma (MM) (n=41), non-Hodgkin's lymphoma (NHL) (n=16: DLBCL 12, FL 2, WM 1, MCL 1), AML (n=10) and myelofibrosis (n=2). Patients had a median of 3 (range 0-8) prior lines of therapy and 29 (42%) had at least 4 prior lines. 28/68 (41.2%) were refractory to the last treatment and 1 patient with newly diagnosed MM was enrolled. A median of 4 (range 0.3-25) cycles of treatment were completed with a median duration of treatment of 3.7 months (range: 0.2-24.4). Treatment was discontinued due to disease progression (36, 62.1%), toxicity (8, 13.8%), proceeding to ASCT (11, 19%), patient choice (2, 3.4%), completed treatment (1, 1.7%). 11 patients are on-going. Following trial discontinuation, 40 (71.4%) received further treatment (clinical trials (n=5), standard therapy (n=24), ASCT (n=11)), 2 (3.6%) were managed expectantly and 14 (25%) palliated.
17 (25%) developed grade 3-4 AEs. 30.9% had dose interruptions for 7 or more days, and 77.9% maintained their planned dose throughout. For those completing 1 or more cycle (n=61, 57 were evaluable), the clinical benefit rate (SD or more) was 68.4% (SD 5 (8.8%), MR 7 (12.3%), PR 15 (26.3%), VGPR 10 (17.5%), CR 2 (3.5%)) with an overall response rate (PR or more) of 47.4%. 18 (31.6%) were refractory to trial treatment.
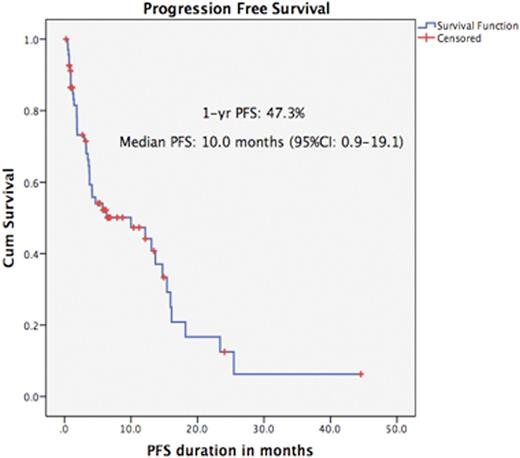
With a median follow-up of 9.6 months (range: 0.2-44.6), the median progression free survival (PFS) was 10.0 months (95%CI: 0.9-19.1 months). The median overall survival was 31.1 months (95%CI: 20.4-41.8) The 90 day mortality rate was 12.8%.
In univariate analysis, LDH and disease types were significant for PFS and OS. However, in multivariate analysis albumin and disease type were independent predictors of OS but not PFS. Serum albumen of >35g/L was associated with an improved OS (HR 0.21, 95%CI 0.06-0.82, p=0.024). Age, performance status, Hb, LDH were not predictive in multivariate analysis. MM was associated with a better PFS and OS over other disease types (see table).
Conclusions: These data demonstrate the wide range of PFS and OS to phase I/II studies according to disease type. Patients with MM had better outcomes and were more likely to access subsequent treatments. Most NHL patients had refractory high grade disease but still had a median OS of 17.9 months. Those with refractory AML/ myelofibrosis had a poor outcome, highlighting a clear unmet need. The PFS and OS for MM and NHL patients was encouraging. Serum albumin was predictive of OS across all disease groups. Whilst such parameters are not primary endpoints for early phase trials, these data indicate their potential clinical benefit. Stratification according to molecular profiles may further improve outcomes.
Yong:Autolus Ltd: Equity Ownership, Patents & Royalties: APRIL based chimeric antigen receptor; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal