Abstract
Introduction: The selection of an allogeneic hematopoietic stem cell transplant (allo-HSCT) donor is highly dependent on human leukocyte antigen (HLA) allele profile matching with that of the intended recipient to minimize graft-related complications and improve post-transplant outcomes. Although ABO compatibility simplifies recipient transfusion needs, transplantation of an ABO incompatible graft has been identified not to have a significant impact on marrow engraftment or recipient survival. We completed an audit of adult allo-HSCT recipients of the Alberta Bone Marrow and Blood Cell Transplant Program to evaluate the impact of ABO incompatibility on engraftment in our allo-HSCT recipient population.
Methods: A retrospective review including all adult allo-HSCT recipients between January 1, 2008 and January 1, 2015 was performed. Data was obtained from review of cellular therapy laboratory electronic records, with blood group confirmation by the transfusion medicine laboratory information system. Statistical calculations were completed using the unpaired t test.
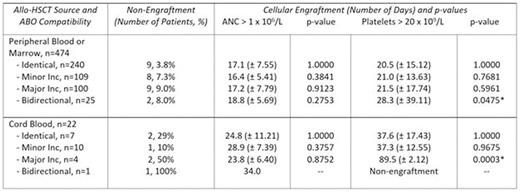
Results:A total of 513 adult patients underwent 528 allo-HSCT procedures (493 peripheral blood, 12 marrow, 23 cord blood). The mean HSCT recipient age was 46 (range 17-66) with 291 (57%) male recipients. The most common HSCT indication was acute myeloid leukemia. All allo-HSCT recipients received myeloablative conditioning. A total of 91% of recipients were conditioned with a regimen of Fludarabine-Busulfan-ATG, with or without total body irradiation. ABO compatibility status for allo-HSCT procedures included the following: 264 (50%) ABO identical grafts, 125 (24%) grafts with a minor incompatibility, 113 (21%) grafts with a major incompatibility, and 26 (5%) grafts with bidirectional incompatibility. HLA matching data was available for 447 allo-HSCT procedures. A total of 350 (78%) patients were recipients of fully HLA matched grafts (340 peripheral blood, 9 marrow, 1 cord blood). Taking into consideration ABO compatibility status, 10/10 HLA matched peripheral blood or marrow grafts were provided to 89% of ABO identical graft recipients, 77% of recipients each with a minor or major incompatibility, and 65% of recipients with a bidirectional incompatibility. Cellular engraftment including the number of days until absolute neutrophil count (ANC) > 1.0 x 106/L and platelet count > 20 x 109/L was available for 496 (94%) of all transplant procedures. A total of 34 transplant recipients did not successfully engraft one or both cell lines. A summary of recipient engraftment data for each category of ABO matching according to stem cell source appears in Table 1.
Conclusion:In our study population, the risk of non-engraftment is lowest in recipients of ABO identical peripheral blood or marrow source donor stem cells. Time to cellular engraftment following allo-HSCT transplant with peripheral blood or marrow source stem cells of minor or major ABO incompatibility is similar to that of an ABO identical donor, while platelet engraftment appears to be prolonged in the setting of a bidirectional incompatibility. However, the small number of recipients of grafts with a bidirectional incompatibility and the large standard deviation affects our confidence in this result.
The impact of ABO matching on engraftment appears to be the greatest in cord blood transplants. The risk of cellular non-engraftment is variable among all ABO compatibility categories, though the time to platelet engraftment is significantly prolonged in recipients of grafts with major ABO or bidirectional incompatibility. These findings are limited by our small cord blood recipient population and the presence of some degree of HLA mismatching in nearly all recipients of cord blood transplants evaluated. Further study is required in larger populations of cord blood transplant recipients to better understand the impact of ABO compatibility status on marrow engraftment, together with variables including the cellular composition of the cord blood graft and host immune factors.
Rate of non-engraftment and days to neutrophil and platelet engraftment following allo-HSCT according to ABO compatibility, including standard deviation and statistical calculation (*indicates statistically significant value).
Rate of non-engraftment and days to neutrophil and platelet engraftment following allo-HSCT according to ABO compatibility, including standard deviation and statistical calculation (*indicates statistically significant value).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal