Abstract
Background: Antithymocyte globulin (ATG) is usually included in conditioning regimens in hematopoietic stem cell transplantation (HSCT) from HLA mismatched donors to reduce the incidence of graft versus host disease (GVHD) and rejection. In this study, we intended to assess the clinical impact of ATG dose in HSCT from HLA mismatched donors.
Patients and Methods: We retrospectively analyzed 268 consecutive patients with acute myeloid leukemia (n=159), acute lymphoblastic leukemia (n=49) or myelodysplastic syndrome (n=60) receiving busulfan based conditioning and HSCT with HLA mismatched sibling, unrelated, or haplo-identical family donors in five centers of Korea between 2005 and 2015.
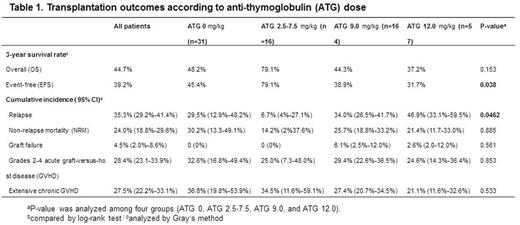
Results: The median age was 44.5 (range, 15-75) years. Fourty-two patients were treated with myeloablative conditioning and 226 patients with reduced intensity conditioning regimen. ATG at a total dose of 0 (n=31), 2.5-7.5 mg/kg (n = 16), 9 mg/kg (n = 164) or 12mg/kg (n=57) was given to patients according to protocol. 140 patients received HSCT in complete remission and 73 patients in relapse or refractory status. The median follow up duration for surviving patients was 33.8 (range, 2.0-100.6) months. The 3-year overall survival (OS) and event free survival (EFS) rate for all patients was 44.7% and 39.2% There was no significant difference of OS according to ATG dose (P=0.153), but ATG dose of 2.5-7.5mg/kg was significantly associated with better EFS than 0, 9 mg/kg, or 12mg/kg of ATG groups (79.1% vs. 45.4%, 38.9%, and 31.7%, respectively, P=0.038) (Table 1). In addition, ATG dose significantly affected on relapse (P=0.046). The relapse incidence in patients receiving 2.5-7.5 mg/kg of ATG was lower compared to those given 0, 9, or 12 mg/kg of ATG (6.7% for 2.5-7.5mg/kg of ATG group vs. 29.5%, 34.0%, and 46.9% for 0, 9, and 12 m/kg of ATG groups, respectively). The incidences of acute GVHD grades II-IV were 32.6%, 25.0%, 29.4% and 24.6% in patients receiving 0. 2.5-7.5, 9, and 12 mg/kg of ATG, respectively. The incidence of extensive chronic GVHD tended to be lower according to the increase of ATG dose (36.8%, 34.5%, 27.4%, and 21.1% for 0. 2.5-7.5, 9, and 12 mg/kg of ATG dose, respectively).In multivariate analysis, ATG dose of 9 mg/kg (P=0.023) and 12 mg/kg (P=0.036) was associated with shorter EFS compared to 2.5-7.5 mg/kg of ATG.
Conclusion: 9mg/kg of ATG dose or more in HLA mismatched busulfan based HSCT with HLA mismatch donors increased the risk for relapse and reduced the EFS.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal