Abstract
Background: The results of the primary endpoint of the E2906 North American Intergroup phase 3 trial - demonstrating significantly inferior OS after single agent clofarabine versus standard intensive daunorubicin & cytarabine induction and consolidation - strongly support the use of intensive therapy for patients age ≥60 years with newly-diagnosed AML who are fit for treatment. This result was achieved despite similar CR rates (including CRi - CR with incomplete blood count recovery) and similar early (30- & 60-day) induction mortality between study arms, raising the question as to the importance of achieving CR as an endpoint. Therefore, we performed a follow-up analysis to address the factors that contribute to early mortality, the necessity for 2nd induction cycle to achieve CR, and to assess the survival impact of the quality of remission.
Methods: E2906 design, study population and results for the primary objective were reported previously (ASH 2015, abstr. #217a). We performed this updated analysis to evaluate clinical risk factors for early mortality and re-induction, and to assess the impact of response quality on OS. Univariate comparisons were made with Fisher's exact test (category) and t-test (continuous variable), and logistic regression models were used to examine risk factors associated with early mortality, and with requiring a 2nd cycle of induction therapy. We performed a landmark analysis (beginning Day 60 after initiation of therapy) to determine the impact of response quality (CR/CRi, morphologic leukemia-free-state (MLFS), and treatment failure - i.e. residual/refractory AML) on OS. Survival comparisons were performed with log-rank test.
Results: A total of n=727 patients were registered to E2906, the median age is 68 years (range 60-86) and the median follow-up of surviving patients is now 18.3 months. Adjusting for treatment, cytogenetic risk group, FAB subtype, WBC count, and secondary AML, increasing age (p=0.06) and performance status (PS) >1 (p=0.05) were associated with 30-day mortality and only PS >1 (p=0.06) with 60-day mortality. Treatment arm (p=0.003), adverse cytogenetic risk group (p=0.02), increasing age (p=0.03) and baseline WBC <10K (p=0.03) were each independently associated with higher risk of receiving 2nd cycle induction, but there was no difference in CR/CRi (44.2% vs. 42.2%, p=0.58) or in median OS (13.8 versus 12.0 months, p=0.11) for those receiving 1 vs. 2 induction cycles, respectively.
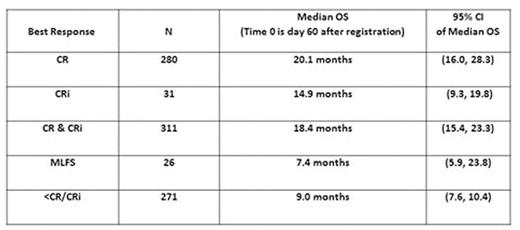
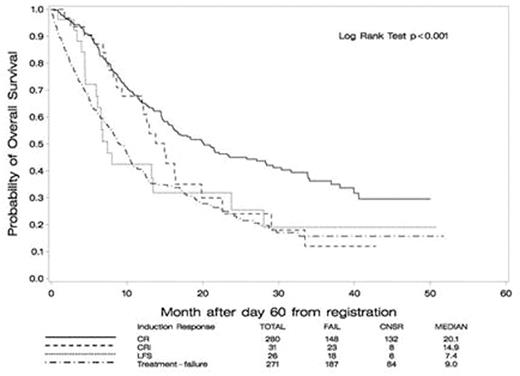
We next performed a landmark analysis (day 60, n=608 evaluable patients) to evaluate OS in relation to the quality of response (Table). We observed an advantage in achieving CR and CR/CRi, although the benefit of CRi diminished greatly after 12 months, and OS after MLFS appeared more similar to treatment failure (Figure, p<0.001). The relative adverse impact of CRi and MLFS (vs. CR) was most pronounced for patients treated with clofarabine, where median OS was shorter for those achieving CRi (12.2m vs. 22.6m standard therapy, p=0.007) and MLFS (6.6m vs. 13.4m standard therapy, p=0.15).
Conclusion: This follow-up analysis confirms the importance of CR regardless of the number of cycles of induction therapy required to achieve remission, and identifies risk factors associated with early mortality and with requiring re-induction. At longer follow-up the OS after CRi appears to be inferior to true CR, particularly after clofarabine. We hypothesize that this may be due to higher rates of minimal residual disease in CRi, and studies to determine this are being performed. MLFS is not associated with a better overall survival than frank treatment failure (i.e. residual disease) and therefore its value as a response category is uncertain. These results highlight the role of standard intensive remission induction therapy with the intent of achieving CR, which remains the standard of care for fit older adults.
Foran:karyopharm: Honoraria; novartis: Honoraria; medscape: Honoraria; Millennium Pharmaceuticals, Inc.: Research Funding; pfizer: Honoraria; boehringer ingelheim: Research Funding; agios: Research Funding; Cellerant: Research Funding. Melnick:Janssen: Research Funding. Altman:Novartis: Honoraria; BMS: Honoraria; Janssen: Honoraria; Syros: Honoraria. Al-Kali:Novartis: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal