Abstract
Chimeric Antigen Receptor (CAR)-modified T cells are a class of immunotherapy, most known for producing durable remissions in B-cell malignancies. To date, On-Target/Off-Tumor effects, systemic cytokine syndromes, and neurotoxicity are some types of toxicities encountered in early CAR T cell therapy trials. Initially, we sought to investigate potential toxicity by a novel, FLT3-targeting CAR T cell (FLT3 CAR). As a tumor-associated antigen, FLT3 is expressed on AML, ALL and MLL, as well as non-malignant hematopoietic subsets, warranting evaluation for On-Target toxicity.
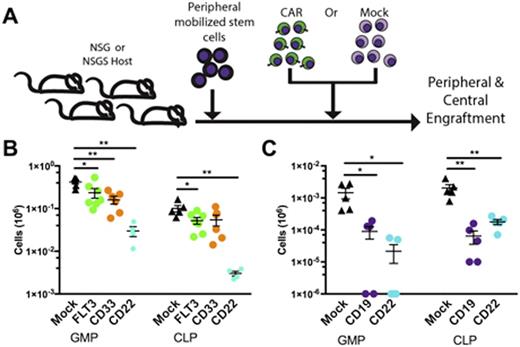
To examine this, human peripheral mobilized CD34+ stem cells were engrafted into either NSG or NSGSÑan NSG-derived knock-in expressing human interleukin-3, GM-CSF, and stem cell factorÑimmunodeficient murine stains. After establishing hematopoietic xenografts and allowing for reconstitution of circulating human myeloid cells, we treated mice with either FLT3 CARs or mock-transduced T cells derived from a marrow-autologous donor [Figure 1A]. In the presence of FLT3 CARs, we observed loss of circulating mature monocytes compared to mock T cell treated controls. To elucidate if myeloid loss originated from an On-Target toxicity towards a FLT3 expressing progenitor or by some other mechanism, we treated marrow-humanized mice with either FLT3 CAR, a lymphoid restricted CD22-targeting CAR, a mature myeloid restricted CD33-targeting CAR or mock transduced T cells. Two weeks following treatment, we analyzed progenitor subsets including human hematopoietic stem cells (HSC), multipotent progenitors (MPP), common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP), maturing marrow granulocytes, common lymphoid progenitors (CLP), and megakaryocyte erythroid progenitors (MEP). Surprisingly, we observed significant marrow loss of CMPs, GMPs, MEPs and CLPs across all mice receiving CARs compared to mice treated with mock transduced T cells alone [Figure 1B]. Absolute numbers of HSCs, MPPs, and total humans cells were equivalent across treatment groups. As the CD19-targeting CAR therapy is most well characterized, we repeated this experiment using CD19 CARs with consistent outcomes [Figure 1C]. Flow cytometric characterization of these progenitor populations confirms that loss still occurs in the absence of target-antigen expression. Taken together, these results suggest that CAR T cell therapy may exert a deleterious effect on specific marrow progenitors through a target antigen-independent mechanism. Furthermore, while a FLT3-CAR On-Target toxicity towards FLT3 expressing progenitors (CLP, GMP, and CMP) can not be fully excluded, an exacerbated progenitor loss compared to target antigen-null subset toxicity in the CD33 CAR and CD22 CAR treated groups was not observed.
Interestingly, prolonged cytopenias are anecdotally observed in Phase I CAR trials, often attributed to heavy pretreatment of enrollees. Local inflammatory cytokine milieu, particularly IFNg, is implicated in marrow suppression in other settingsÑchronic viral infections, donor lymphocyte infusion and disseminated non-tuberculous mycobacterial infections. We hypothesize that prolonged CAR T cell cytokine secretion may exert similar marrow suppression. This could be due to either continuous CAR-engagement with reconstituting antigen-positive progenitors or an intrinsic supraphysiologic basal level of cytokine secretion by CAR T cells. We are currently analyzing cytokine profiles in our humanized murine model to further evaluate this hypothesis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal