Abstract
Background: Ponatinib is a tyrosine kinase inhibitor (TKI) approved for adult patients (pts) with relapsed/refractory CML or Ph+ ALL and those with the BCR-ABL T315I mutation, which is uniformly resistant to other TKIs approved for the treatment of CML and Ph+ ALL. Prior to the availability of ponatinib, resistant pts with the T315I mutation (T315I+) had worse outcomes than those without the mutation; in a matched pair analysis in chronic phase (CP)-CML pts, median overall survival (OS) was 48.4 mos after development of resistance in T315I+ pts versus not reached in those without the mutation (Nicolini FE, et al. Haematologica 2013).
Methods: We evaluated the efficacy and safety of ponatinib in a pooled analysis of a subgroup of CP-CML pts with the T315I mutation (detected in a central laboratory by Sanger sequencing at baseline) from the phase 1 (NCT00660920) and pivotal phase 2 PACE (NCT01207440) trials. In addition, we evaluated the impact of continuation of ponatinib treatment at a 2-yr landmark time point on OS at 1-yr past the landmark in T315I+ CP-CML pts in PACE. The phase 1 trial is an open-label, dose escalation study of ponatinib (starting dose 2-60 mg once daily) in 81 adults with relapsed/refractory hematologic malignancies. PACE is an open-label, single-arm trial of ponatinib (starting dose 45 mg daily) in 449 adults with CML or Ph+ ALL resistant or intolerant to dasatinib or nilotinib or with the T315I mutation. Dose reductions were instructed in Oct 2013 in response to an accumulation of arterial occlusive events (AOEs) reported with longer follow-up across the ponatinib clinical program. Response assessments included major cytogenetic response (MCyR), complete cytogenetic response (CCyR), major molecular response (MMR; assessed in a central laboratory), and molecular response 4.5 (MR4.5). OS and progression-free survival (PFS) data were only collected in PACE; 3-yr outcomes were examined for all evaluable T315I+ CP-CML pts, along with a log-rank test for OS by treatment status as of the 2-yr landmark time point. Exposure-adjusted incidence rates of new AOEs are reported as number of events/100 pt-yrs. Pooled data as of February 2, 2015 is reported here.
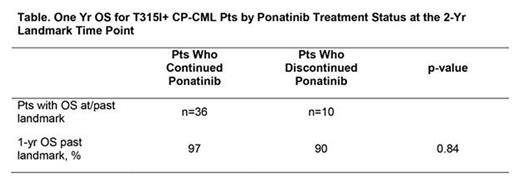
Results: There were 76 T315I+ CP-CML pts included in this analysis (phase 1, n=12; PACE, n=64). At the time of analysis, median duration of follow-up in T315I+ CP-CML pts was 40 (range: 1.5-74) mos; 37 pts (49%) remain on study. Median baseline ponatinib dose intensity was 33 (range: 5-56) mg daily; 25/37 (68%) ongoing pts were receiving 15 mg daily as their current dose as of the data cut-off. Primary reasons for discontinuation in T315I+ CP-CML pts were disease progression [10/76 (13%)], adverse events [AEs; 9/76 (12%)], consent withdrawn [5/76 (7%)], physician/administrative decision [4/76 (5%)], death [3/76 (4%)], lack of efficacy [2/76 (3%)], and other [6/76 (8%)]; criteria for disease progression included death, development of advanced phase CML, loss of CHR (in absence of cytogenetic response) and loss of MCyR. Cumulative response rates in T315I+ CP-CML pts (n=76) were: MCyR, 75%; CCyR, 72%; MMR, 61%, and MR4.5, 37%. In PACE, estimated 3-yr PFS/OS rates for 64 T315I+ CP-CML pts were 60%/78% (medians not reached). One yr post-landmark outcomes for T315I+ CP-CML pts by treatment status at the 2-yr landmark time point are displayed in the table. Most common treatment-emergent AEs (≥40%) in the pooled group of T315I+ CP-CML pts (n=76 phase 1 and PACE) were: rash, 55%; dry skin, 49%; headache, 46%; abdominal pain, 43%; nausea, 41%; and fatigue, 41%. Among these pts, the cumulative incidence of any AOE (grade 3/4) was 32% (20%); by subcategory: cardiovascular 20% (15%), cerebrovascular 12% (5%), and peripheral vascular 13% (8%) events. Two T315I+ CP-CML pts had grade 5 AOEs. The exposure-adjusted incidence rate of new AOEs in T315I+ CP-CML pts was 12/100 pt-yrs.
Conclusions: Ponatinib continues to provide deep and durable responses with >3 yrs median follow-up in T315I+ CP-CML pts. Survival outcomes with ponatinib treatment in these highly refractory pts were high overall and compare favorably with those observed in T315I+ CP-CML pt populations prior to the availability of ponatinib. Although pt numbers are limited for the landmark analysis, continuation of ponatinib treatment at 2 yrs was associated with a trend for improved OS, where data continue to mature. Updated data in all T315I+ pts will be presented.
Study sponsor: ARIAD Pharmaceuticals, Inc.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Talpaz:Incyte Corporation: Other: Travel expense reimbursement, Research Funding; Novartis: Research Funding; Ariad: Other: Expense reimbursement, travel accomodation expenses, Research Funding; Pfizer: Consultancy, Other: travel accomodation expenses, Research Funding. Baccarani:ARIAD: Consultancy, Honoraria, Other: travel, accommodations, expenses , Speakers Bureau; BMS: Honoraria, Speakers Bureau; Novartis: Honoraria, Other: travel, accommodations, expenses , Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Mauro:BMS: Consultancy, Honoraria; ARIAD: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria. Hochhaus:Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding. Hughes:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria. Guilhot:ARIAD: Honoraria. Deininger:BMS: Consultancy, Research Funding; Gilead: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CTI BioPharma Corp.: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Ariad: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shah:ARIAD: Research Funding; Daiichi-Sankyo: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Plexxikon: Research Funding. Flinn:Janssen: Research Funding; Gilead Sciences: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; ARIAD: Research Funding; RainTree Oncology Services: Equity Ownership. Lustgarten:ARIAD: Employment, Equity Ownership. Rivera:ARIAD: Employment, Equity Ownership. Santillana:ARIAD: Employment, Equity Ownership. Kantarjian:Amgen: Research Funding; ARIAD: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal