Abstract

Background: Many patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) achieve a sustained deep molecular response (DMR) with frontline nilotinib (NIL) therapy. ENESTfreedom (NCT01784068) is an ongoing phase 2 study evaluating the potential for such pts to stop treatment and remain in treatment-free remission (TFR). Initial results from ENESTfreedom showed that 51.6% of pts who attempted TFR remained off treatment without loss of major molecular response (MMR [BCR-ABL1 ≤ 0.1% on the International Scale, BCR-ABL1IS]) at 48 weeks. Despite the enrollment of pts with established tolerance of NIL, the frequency of adverse events (AEs) decreased during the first 48 weeks of TFR compared with the year prior to stopping treatment (65.8% vs 83.2%, respectively), while AEs related to musculoskeletal pain were more common during TFR (24.7% vs 16.3%, respectively). The quality of life (QOL) of tyrosine kinase inhibitor-treated pts has gained increasing interest in recent years. To evaluate the impact of TFR on QOL, we analyzed patient-reported outcomes prior to and during TFR.
Methods: Pts with CML-CP and ≥ 2 years of frontline NIL therapy (400 to 600 mg/day for the previous ≥ 1 year) who achieved MR4.5 (BCR-ABL1IS ≤ 0.0032%) on NIL were enrolled and entered a 1-year consolidation phase, during which they continued NIL with RQ-PCR assessments every 12 weeks. Pts with sustained DMR during the consolidation phase (no assessment worse than MR4 [BCR-ABL1IS ≤ 0.01%], ≤ 2 assessments between MR4 and MR4.5, and MR4.5 in the last assessment) entered the TFR phase and stopped treatment. Pts with loss of MMR during the TFR phase reinitiated NIL treatment (reinitiation phase). At specified time points, pts completed the MD Anderson Symptom Inventory for CML (MDASI-CML, in which pts rate the levels of severity and interference with daily life for a defined set of symptoms on a scale from 0 to 10, with 0 indicating the lowest severity/interference). At each time point, pts also completed the EQ-5D-5L questionnaire, in which they report the presence/absence and severity (slight, moderate, severe, or extreme) of problems related to mobility, self-care, usual activities, anxiety/depression, and pain/discomfort and rank their overall level of health from 0 to 100 using the EQ VAS scale, with 0 indicating the poorest level of health.
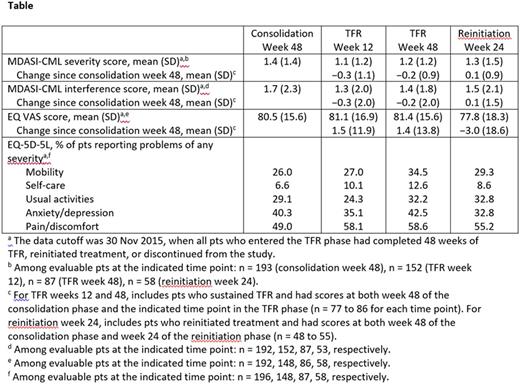
Results: Among 215 pts enrolled, 190 remained in sustained DMR during the consolidation phase and entered the TFR phase. Mean MDASI-CML severity and interference scores and EQ VAS scores, respectively, among pts who completed each questionnaire were 1.4, 1.7, and 80.5 at week 48 of the consolidation phase; 1.1, 1.3, and 81.1 at week 12 of the TFR phase; and 1.2, 1.4, and 81.4 at week 48 of the TFR phase (Table). Among pts who sustained TFR and who had scores at both week 48 of the consolidation phase and week 12 or 48 of the TFR phase, no impact of stopping treatment on MDASI-CML or EQ VAS scores was detected. Among evaluable pts who lost MMR during the TFR phase and reinitiated NIL, mean scores at 24 weeks after treatment reinitiation were 1.3 (MDASI-CML severity), 1.5 (MDASI-CML interference), and 77.8 (EQ VAS). Among pts evaluable at both week 48 of the consolidation phase and week 24 of the reinitiation phase, mean scores were similar at both time points.
Among pts who completed the EQ-5D-5L questionnaire, the proportions reporting problems (of any severity) at week 48 of the consolidation phase, weeks 12 and 48 of the TFR phase, and week 24 of the reinitiation phase tended to be similar. The proportion of pts reporting problems with anxiety/depression was lowest during the reinitiation phase (Table).
Conclusion: Minimal changes in patient-reported outcomes were observed after stopping treatment. This may be related to pts having a relatively high QOL prior to stopping treatment, given that they had tolerated ≥ 2 years of NIL prior to enrollment. These data suggest that the higher frequency of musculoskeletal pain-related AEs in the TFR phase did not substantially impact pts' QOL; however, only a subset of pts were evaluable for changes in reported outcomes over time. Although many pts have fears about TFR, reported levels of anxiety/depression were similar before and after stopping treatment but decreased among pts who reinitiated treatment.
Hochhaus:BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; ARIAD: Honoraria, Research Funding. Casares:Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Speakers Bureau; Ariad: Consultancy, Speakers Bureau. Stentoft:Pfizer: Research Funding; Ariad: Research Funding; Bristol-Myers-Squibb: Research Funding; Novartis: Research Funding. Conneally:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. García-Gutiérrez:Ariad: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Gattermann:Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Other: travel, accomodation expenses, Research Funding. Wiktor-Jedrzejczak:Sandoz: Consultancy; BMS: Research Funding; Novartis: Consultancy, Research Funding; Janssen-Cilag: Consultancy; Angelini: Consultancy; Novartis: Consultancy, Research Funding; Celgene: Consultancy; Amgen Inc.: Research Funding. Le Coutre:Ariad: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Other: travel, accomodations, expenses; Novartis: Consultancy, Honoraria, Other: travel, accomocations, expenses, Research Funding. Saussele:ARIAD: Honoraria; Novartis: Honoraria, Other: Travel grants, Research Funding; Pfizer: Honoraria, Other: Travel grants; BMS: Honoraria, Other: Travel grants, Research Funding. Giles:Novartis: Consultancy, Research Funding. Radich:Ariad: Consultancy; BMS: Consultancy; Novartis: Consultancy, Research Funding. Ross:BMS: Honoraria; Novartis Pharmaceuticals: Honoraria, Research Funding. Menssen:Novartis Pharma AG: Employment. Deng:Novartis Phamaceuticals Corp.: Employment. Brandt:Novartis: Employment. Gnanasakthy:Novartis: Consultancy, Equity Ownership, Research Funding. Bedoucha:Novartis: Employment. Saglio:Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Ariad: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal