Abstract
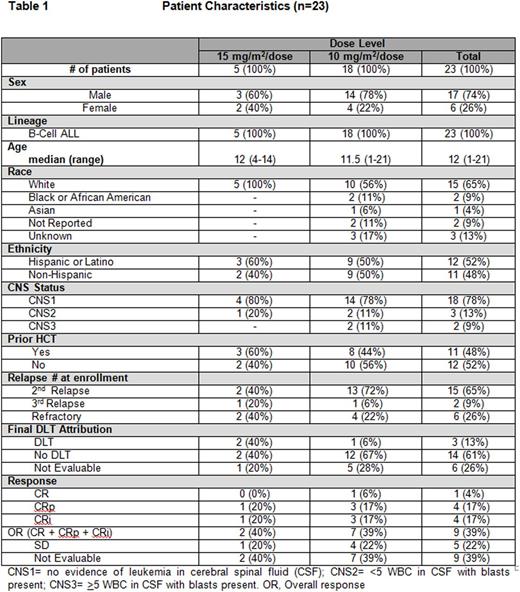
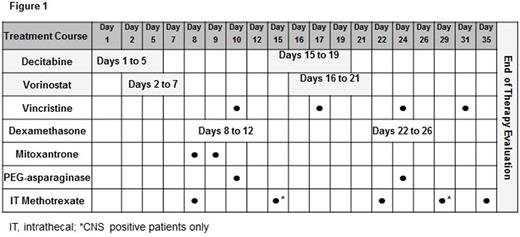
Acquired drug resistance is a major reason for relapse and death in acute lymphoblastic leukemia (ALL). Improving outcomes by targeting mechanisms of acquired drug resistance is a potential solution. DNA methyltransferase inhibitors (DNMTi) and histone deacetylase inhibitors (HDACi) have been shown to reverse drug resistance gene signatures and restore chemosensitivity in relapsed ALL. We report results investigating decitabine (DNMTi) (10-15 mg/m2/dose) and vorinostat (HDACi) (180 mg/m2/dose) with vincristine, dexamethasone, mitoxantrone and PEG-asparaginase (UKALL R3 backbone) for relapsed/refractory ALL. Twenty-three patients with refractory or ≥2nd relapse B-ALL were enrolled (Table 1). Median age was 12 (range, 1-21) years. Toxicity was graded according to CTCAE v4.0. The most common grade (Gr) 3-4 toxicities included hypokalemia (65%), anemia (61%), febrile neutropenia (57%), hypophosphatemia (43%), hyperbilirubinemia (39%), thrombocytopenia (39%), neutropenia (35%), sepsis (30%), lung infection (30%) and infection/infestations-other (30%). Non-hematologic dose limiting toxicity (DLT) was defined as any Gr 3 or 4 adverse event that was at least possibly attributed to decitabine and/or vorinostat with the exception of nausea (Gr 3), vomiting (Gr 3), transaminase elevation that returned to Gr ≤1 or baseline prior to the end of the course, fatigue, fever/infection, electrolyte abnormalities that were transient and not associated with clinical sequelae, albumin, alkaline phosphatase, GGT and anorexia. Hematologic DLT was defined as an absence of peripheral blood count recovery within 6 weeks of starting chemotherapy. Any patient who experienced a DLT after receiving at least 1 dose of decitabine or vorinostat was evaluable for DLT. Patients who did not experience a DLT had to receive a pre-determined amount of protocol therapy as outlined in the study to be evaluable for DLT. Seventeen of 23 patients were evaluable for DLT. Three patients experienced DLTs as follows: Gr 3 cholestasis, Gr 3 steatosis, Gr 4 hyperbilirubinemia (n=1); Gr 4 seizure, Gr 4 somnolence, Gr 3 delirium (n=1); and Gr 3 pneumonitis, Gr 3 hypoxia, Gr 3 hyperbilirubinemia (n=1). The study was suspended and amended after the first 5 patients enrolled (decitabine, 15mg/m2), due to significant infectious toxicities [4 of 5 patients reporting invasive fungal infections: C. kruseii (n=2), C. lusitaniae (n=1), and C. guillermondii (n=1)] and two DLTs (reported above). The treatment schema was modified to lower the dose of decitabine (10mg/m2/dose) and to reduce the duration from days 1-7/15-21 to 1-5/15-19. The duration of vorinostat was also reduced from days 3-10/17-24 to 2-7/16-21 (Figure 1). In addition, anti-fungal prophylaxis with either an echinocandin or amphotericin agent was required. Despite these dose modifications, infectious complications remained common with 17/23 (74%) patients reporting Gr ≥3 infections including invasive fungal disease in 35% (8/23). In analyzing all 23 patients, the complete response rate (CR + CR with incomplete blood count recovery) was 39% (9/23). Fourteen patients (61%) were able to complete full protocol therapy and evaluable for response. The remaining 9 patients did not complete therapy, primarily due to treatment-related toxicities, and were not evaluable. Nine of the 14 patients (64%) achieved a complete response with the remaining 5 reporting stable disease (36%). Minimal residual disease (MRD) testing via flow cytometry was performed on 7 of 9 patients who achieved a CR with a median level of 0.087% MRD (range, 0.00% to 1.6%) detected. Overall survival at 6 months was 35.1% (95% CI, 16.0 - 54.9) and 23.4% (95% CI, 7.8 - 43.6) at 1-year. Death was reported in 18/23 (78%) patients at a median of 10 weeks from study start (range, 3-138). Cause of death was attributed to refractory leukemia (n=8), treatment related toxicity (n=5), infection (n=3) and transplant-related toxicity (n=2). Despite encouraging response rates including low MRD, the combination of decitabine and vorinostat with intensive chemotherapy in this patient population was determined not to be feasible. A future follow-up study with a less intensive chemotherapy backbone is needed to determine if these two promising epigenetic agents can be successfully incorporated into the treatment of pediatric ALL. This study is registered at http://www.clinicaltrials.gov as NCT01483690.
Wayne:Kite Pharma: Honoraria, Other: Travel support, Research Funding; Spectrum Pharmaceuticals: Honoraria, Other: Travel Support, Research Funding; Pfizer: Consultancy, Honoraria, Other: Travel Support; Medimmune: Honoraria, Other: Travel Support, Research Funding; NIH: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal