Abstract
Introduction: CD19 antibody therapy may represent an attractive treatment option in pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL). Since conventional CD19 antibodies have failed in clinical trials, different strategies are evaluated to target CD19 more efficiently. Beside the bispecific T cell engager blinatumomab and chimeric antigen receptor T-cells, antibody drug conjugates and antibodies with engineered fragments crystallisable(Fc)for improved effector cell engagement are under investigation. Here, we demonstrate the efficacy of Fc-engineered CD19 antibodies in minimal residual disease (MRD) xenograft models of pediatric BCP-ALL. We further suggest an important contribution of macrophages for this type of therapy.
Methods: An Fc-engineered CD19 antibody carrying amino acid mutations S239D/I332E (CD19-DE) and its native CD19-IgG1 variant were generated according to published sequences. CD19-DE was analyzed in patient-derived leukemia xenografts from infants with MLL-rearranged BCP-ALL, which were established by intrafemoral transplantation of 100 cells per animal in NOD-SCID-gamma-/- (NSG) mice lacking a functional lymphatic compartment. CD19-DE was injected intraperitoneally (1 mg/kg on days +1, +3, +6, +10, +13, and every 21 days thereafter; MRD-model). In some experiments leukemia development (defined as >1% peripheral blasts; overt leukemia model) was awaited before CD19-DE was applied alone, or in combination with a regimen mimicking standard induction chemotherapy (Dexamethasone days 1-5, Vincristine day 1 and PEG-Asparaginase day 1 every 28 days). MRD status was determined by analysis of bone marrow DNA for patient-specific immunoglobulin (Ig)-rearrangements and MLL-fusion genes by polymerase chain reaction. In order to test the role of macrophages as effector cells, macrophages were depleted by intraperitoneal injection of liposomal clodronate. In vitro phagocytosis of BCP-ALL primary cells from xenografts was determined by fluorescence microscopy. For that purpose, macrophages were differentiated from human monocytes with macrophage colony-stimulating factor and BCP-ALL cells were labelled with a fluorescent membrane dye.
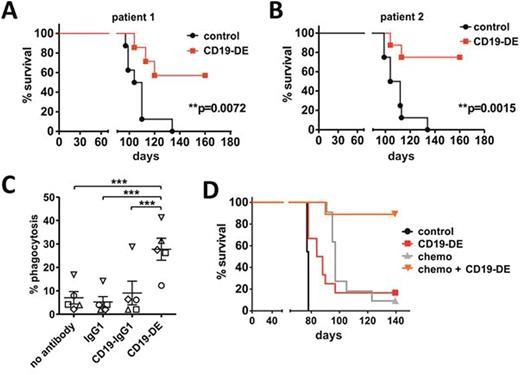
Results: CD19-DE was efficient in prolonging the survival of NSG xenografts of two patients tested in the MRD-model (p = 0.0072 and p = 0.0015, Kaplan-Meier log rank test, Figure A/B). Interestingly, analyses of bone marrow DNA from the surviving mice for two patient specific Ig-rearrangements and the respective MLL-fusion revealed that 4/5 mice were MRD-negative by Ig-rearrangement and 3/5 mice were MRD-negative by MLL-fusion. In order to identify effector mechanisms, antibody therapy was performed in the MRD-model with and without depletion of macrophages. Macrophage depletion in vivo resulted in a reversal of the beneficial effects of CD19-DE as measured by increases in splenic volumes and percentage of human blasts in the bone marrow, suggesting an important role for macrophages in CD19 antibody therapy. CD19-DE was next analyzed for its ability to engage human macrophages in phagocytosis assays with primary BCP-ALL blasts from xenograft mice in vitro. CD19-DE effectively triggered phagocytosis of BCP-ALL cells, whereas a corresponding native CD19 IgG1 antibody did not (ANOVA, p < 0.0001, Figure C; data points indicate results with macrophages from 5 different donors), which emphasizes the importance of Fc-engineering for the efficacy of CD19 antibodies. Finally, therapy with CD19-DE was initiated in the overt leukemia model alone and in combination with chemotherapy. CD19-DE was still efficient in prolonging survival as compared to control animals (p = 0.0003, Figure D), but the effects were less pronounced. Importantly, the combination of antibody therapy and cytoreductive chemotherapy resulted in prolonged survival of 90% of the animals as compared to control animals (p < 0.0001) or animals treated with chemotherapy alone (p = 0.0054; Figure D).
Conclusion: These preclinical in vivo data obtained in xenograft models of BCP-ALL suggest a high therapeutic potential of Fc-engineered CD19 antibodies and indicate an important role for macrophages in that context. Administration of Fc-engineered CD19 antibodies in an MRD situation or concomitant application of the antibody and cytoreductive chemotherapy may represent promising approaches in the therapy of pediatric BCP-ALL.
Gramatzki:Janssen: Other: Travel/Accommodation/Expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal