Abstract
Background: For patients (pts) with severe aplastic anemia (SAA) lacking an HLA identical donor, outcomes of hematopoietic stem cell transplantation (HSCT) using unrelated cord blood (UCB) units or haplo-identical donors (HDs) have historically been associated with high graft failure rates and poor survival. In an ongoing clinical trial at the NHLBI, we have observed excellent engraftment (100%) and survival (91%) in SAA pts (n=27) receiving a transplant that co-infuses a single UCB unit with CD34-selectedCD3-depletedcells from a haplo-identical relative. Although cord myeloid engraftment(defined as cord ANC >500/μL) occurred at<day 100 in the majority of pts, a significant fraction of pts had delayed (>day 100) or no cord myeloid engraftment. In this analysis, we investigated factors that may have impeded cord myeloid engraftment following UCB/HD transplantation.
Methods: Flow-based NK cell phenotyping using a BD Fortessa II instrument was performed on blood obtained pre-transplant from HDs used for the first 18 SAA pts undergoing UCB/HD transplantation. Lineage specific chimerism was measured by PCR of microsatellites (PowerPlex 16 HS Systemkit/Promega) using DNA from flow sorted cells (BD FACSAria) collected multiple time points post-transplant.KIR-ligand incompatibility in the HD vs UCB directionwas defined using high-resolution HLA typing.
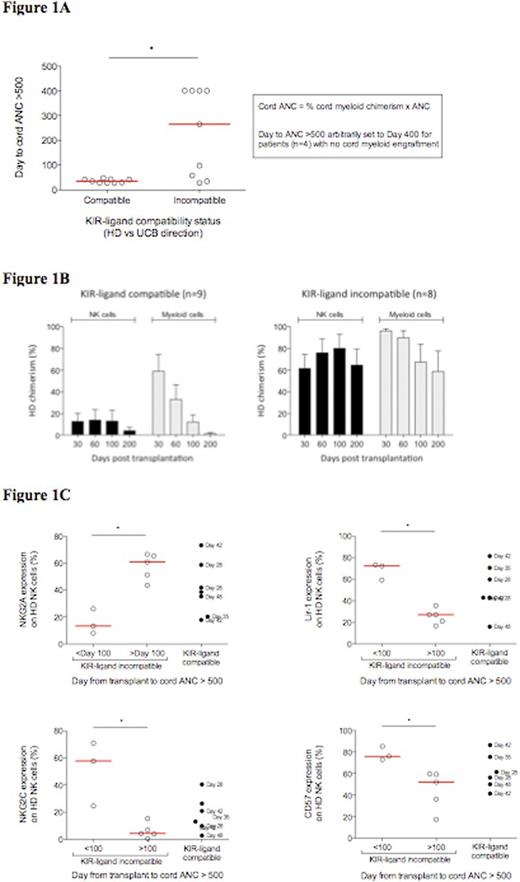
Results: 13/18 (72%) pts had cord myeloid engraftment before day 100 while 5/18 (28%) had delayed or no cord myeloid engraftment. Remarkably, delayed or no cord myeloid engraftment occurred exclusively in pts transplanted with KIR-ligand incompatibility in the HD vs UCB direction (n=9) (Figure 1A). In contrast, all 9 pts transplanted with KIR-ligand compatibility in the HD vs UCB direction achieved cord myeloid engraftment by ²day 48 (median day 35) post-transplant. Chimerism analysis performed on blood obtained 30+ days post-transplant revealed NK cell chimerism was ³ 90% cord in origin in all 9 pts transplanted with KIR-ligand compatible grafts. In contrast, amongst the 9 pts receiving a KIR-ligand incompatible transplant, NK cell chimerism was predominantly HD in origin with only a minor fraction of cord NK cells detected 30-200 days post-transplant (Figure 1B). Predominant HD NK cell chimerism in pts receiving a KIR-ligand incompatible transplant was associated with lower degrees of cord myeloid chimerism compared to KIR-ligand compatible recipients. Further analysis of the KIR-ligand incompatible cohort revealed distinct heterogeneity in the time to cord myeloid engraftment (Figure 1A). Although delayed or no cord myeloid engraftment was observed in 5/9 recipients of KIR-ligand incompatible transplants, 4/9 pts in this cohort had cord engraftment at a similar time as pts transplanted with KIR-ligand compatible grafts (median 35 vs. 35 days). This variability in time to cord myeloid engraftment was not associated with stem cell dose, degree of HD NK cell chimerism, type of KIR-ligand incompatibility or KIR haplotype. However, we observed a strong correlation between the proportion of naive NK cells in circulation of HDs before stem cell mobilization with delayed or no myeloid cord engraftment (Figure 1C). With the exception of one patient who had failed HD engraftment, only transplants of CD34+ cells from HDs with a predominantly naive NK cell repertoire, expressing high frequencies of the NKG2A receptor concomitant with low frequencies of NKG2C, Lir-1 and CD57 resulted in delayed or no cord myeloid engraftment (p<0.05).
Conclusions: Our study provides the first evidence that NK cells from engrafting CD34+ cells from selected HDs can significantly delay or completely inhibit cord myeloid engraftment following UCB/HD transplantation. Suppression of cord hematopoiesis appears to be restricted to NK cells originating from HDs withHD vs UCB KIR-ligand incompatibility who have a large naive NK cell repertoire in their circulation prior to stem cell mobilization. The myelosuppressive effects of these NK cells are consistent with recentlypublished data showing a naive NK cell repertoire in stem cell donors predicts a reduced risk of AML relapse post-allogeneic HSCT.Further studies defining the mechanisms through which naive NK cells suppress cord hematopoiesis followingUCB/HDtransplantation could shed insights into methods to optimize NK cell mediated graft-vs-leukemia followingallogeneicHSCT of myeloid leukemias.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal