Abstract
Introduction: Primary graft failure or delayed engraftment is a well-studied complication of allogeneic hematopoietic stem cell transplantation (HSCT). According to previous studies, the following factors are associated with primary graft failure or delayed engraftment: underlying disease, disease status, human leukocyte antigen (HLA) disparity between recipient and donor, type of conditioning regimens, stem cell source, and stem cell dose. As engraftment failure or delayed engraftment frequently brings uncontrollable infectious events for allogeneic HSCT recipients, the prevention of primary engraftment failure and the promotion of early engraftment is critically required. Splenomegaly is associated with primary engraftment failure and poor overall survival (OS) after allogeneic HSCT in patients with myelofibrosis. As patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) sometimes develop splenomegaly, we investigated the effect of splenomegaly on engraftment kinetics in patients with AML and MDS.
Patients and methods: A retrospective case analysis was performed for 87 patients with AML or MDS who were treated with allogeneic HSCT at our hospitals from April 2010 to March 2016. Two patients without computed tomography data just before allogeneic HSCT were excluded. The remaining 85 patients were included in the analysis. Spleen volume was evaluated using iPlan. We set the primary endpoint as neutrophil engraftment (>500/mL) at 28 days after allogeneic HSCT. We also evaluated OS, progression-free survival (PFS), cumulative incidence (CI) of non-relapse mortality (NRM) and CI of relapse (CIR). To provide simple risk stratification, we also employed survival classification and regression tree (CART) analysis, and divided study patients into two risk groups. To evaluate the impact of spleen volume on the endpoint, we employed multivariable Cox regression analysis for OS and PFS and Fine and Gray analysis for engraftment; CI of NRM and CIR were calculated using spleen volume as the continuous variable. Adjusted covariates were as follows: age, sex, disease, disease status, stem cell source, and HLA mismatch.
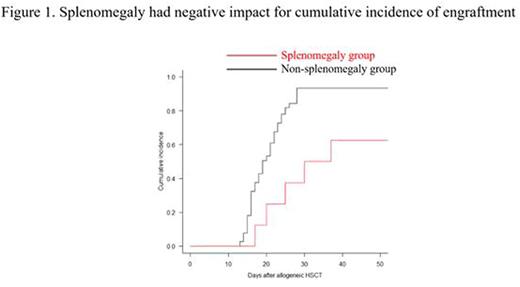
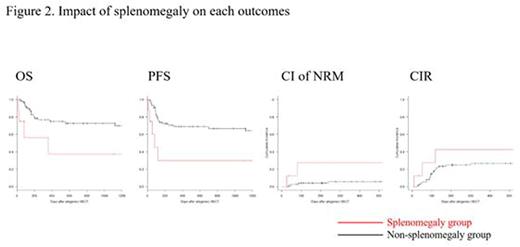
Results: Median spleen volume was 145 cm3 (38-1661 cm3) among the study patients. The survival CART analysis revealed that the discriminating spleen volume to discern engraftment was 320 cm3. Thus, we defined patients with a spleen volume above 320 cm3 as part of the splenomegaly group, and the remaining patients as part of the non-splenomegaly group. The neutrophil engraftment rate at 28 days after allogeneic HSCT was 37.5% and 93.5% (p = 0.021) in the splenomegaly and non-splenomegaly groups, respectively (Figure 1). OS at 2 years was 37.5% and 72.6% (p = 0.011), PFS at 2 years was 30% and 67% (p = 0.005), CI of NRM at 1 year was 27.5% and 5.6% (p = 0.044), and CIR at 1 year was 42.5% and 26.5% (p = 0.34) in the splenomegaly non-splenomegaly groups, respectively (Figure 2). To further assess the influence of spleen volume on engraftment and other outcomes previously described, we performed multivariate Cox regression analysis or Fine and Gray analysis. The adjusted HR (95% CI) for spleen volume per 100 cm3 was 0.79 (0.68-0.92, p = 0.003) on engraftment, 1.23 (1.04-1.47, p = 0.017) on OS, 1.27 (1.08-1.5, p = 0.005) on PFS, 1.64 (1.09-2.4, p = 0.016) on CI of NRM, and 0.96 (0.83-1.1, p = 0.59) on CIR.
Conclusion: Our study showed that spleen volume predicts poor engraftment rate and poor PFS due to increased NRM. Patients with splenomegaly were at a higher risk of engraftment failure or delayed engraftment and may be considered for spleen irradiation before allogeneic HSCT.
Disclosure of Interest: We declare no conflicts of interest.
Ishikawa:Mundipharma KK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal