Abstract
Central nervous system lymphoma (CNSL) is a lymphoid malignancy in which tumors from lymph tissue start in the brain, spinal cord, eye, and/or meninges (primary CNSL) or present as a result of metastasis from initial systemic sites to the CNS (secondary CNSL). The most common CNS lymphomas (about 90%) are B-cell lymphomas. The incidence of primary CNS lymphoma has been increasing over the past 20 years. Multifocal lesions are common. CNS lymphomas carry a worse prognosis than systemic lymphoma. Only a few chemotherapeutic drugs can cross and achieve a therapeutic concentration in the CNS. Therefore, effective treatment is limited and the outcome of disease in relapsed or refractory setting is poor.
Recent studies show that intraventricular delivery of rituximab in CNS lymphomas is well tolerated. T cell products that are genetically engineered with chimeric antigen receptors (CARs) targeting CD19 have broad application for adoptive therapy of B cell lineage malignancies and have shown tremendous potential in treatment of systemic lymphoma. In all CD19CAR T cell trials, T cell products are administrated intravenously. CD19CAR T cell trafficking in cerebrospinal fluid (CSF) is frequently reported but most if not all protocols exclude patients with active CNS involvement. In this study, we set out to investigate the feasibility and efficacy of the use of CD19CAR T cells to treat CNSL.
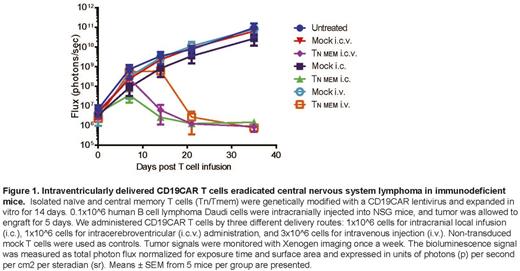
Methods and Results: Isolated naïve and central memory T cells (Tn/Tmem) were genetically modified with CD19CAR lentivirus and expanded in vitro for 14 days. 0.1x10^6 human B cell lymphoma Daudi cells were injected intracranially into NSG mice. Tumor was allowed to engraft for 5 days. We administered CD19CAR T cells via three different delivery routes: intracranial local infusion with 1x10^6 CD19CAR T cells (i.c), intracerebroventricular (i.c.v) administration with 1x10^6 cells to bypass the blood-brain barrier and target tumor throughout the entire CNS, and intravenous injection (i.v) with 3x10^6 cells. We repeatedly observed in 2 separate experiments (N=5 mice in each experiment) that both a single i.c infusion and a single i.c.v delivery of CD19CAR T cells were able to completely eradicated CNS lymphoma in all mice by day 14 post CAR T cell infusion; and that a single dose of i.v infusion induced significant anti-CNSL activity with a slightly delayed response as compared to i.c and i.c.v treatment and all mice achieved complete remission 21 days post T cell infusion. CAR T cells were detected in peripheral blood obtained from retro-orbital bleeding, not only in the i.v treated mice, but also in i.c.v treated mice 28 days after CAR T cell infusion, suggesting that i.c.v not only controls CNSL but may also play a role in immune surveillance for systemic tumors. To confirm this, we established an NSG CNS B cell lymphoma model by also inoculating subcutaneous tumors on the animal's flank, 3 weeks prior to i.c tumor injection into the same mouse. CD19CAR T cells were delivered via i.c.v 5 days after i.c. tumor injection. CAR T cell injection resulted in complete remission of both the brain tumor and the flank tumor 14 days after CAR T cell administration.
In conclusion,intracerebroventricular delivery of CD19CAR T cells is a promising and feasible therapeutic approach for both primary central nervous system lymphoma and systemic lymphoma with concurrent CNS involvement.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal