Abstract
Patients with myelofibrosis (MF) often develop anemia and frequently become dependent on red blood cell transfusions. Elevated levels of hepcidin in MF patients suggest that deregulated iron homeostasis is a driver of anemia in MF (Pardanani et al., Am J Hematol, 2013). A phase 2 study in MF patients demonstrated that momelotinib (MMB) treatment resulted in improvement of anemia (Pardanani et al., Blood, 2013, ASH Suppl). We have demonstrated that in addition to inhibiting JAK1/2, MMB also inhibits the bone morphogenetic protein receptor kinase, activin A receptor type 1 (ACVR1), a key regulator of hepcidin production in hepatocytes (Asshoff et al., Blood, 2015, ASH Suppl). This work supports a model by which the improvement of anemia observed in MF patients with MMB treatment results from the inhibition of ACVR1-mediated hepcidin expression in the liver, increased mobilization of sequestered iron from cellular stores and subsequent stimulation of erythropoiesis.
Here we demonstrate that MMB treatment in anemic rats resulted in a reduction of hepcidin and amelioration of anemia; whereas ruxolitinib dosing affected neither hepcidin nor anemia. Short-term MMB oral treatment in anemic rats resulted in a dose responsive inhibition of serum hepcidin induction with complete suppression at 25 mg/kg QD, 82% inhibition at 10 mg/kg QD, and 55% inhibition at 5 mg/kg QD (Table 1). Interestingly, only the two higher doses show significant time above EC50 levels, whereas the 5 mg/kg dose (which most closely approximates MMB human clinical exposures) only shows significant time above EC20. This suggests that systemic drug concentrations alone may not accurately predict activity in the model as 5 mg/kg demonstrated a 55% reduction in serum hepcidin. In a rat quantitative whole body autoradiography tissue distribution study, MMB levels have been demonstrated to be 3-4 fold higher in the liver relative to blood (Figure 1). Thus, the significant inhibiton of hepcidin levels in animals dosed with 5 mg/kg MMB may be due to higher concentrations in the liver. MMB concentrations would be expected to be higher in the liver relative to blood in humans as well.
Our results provide preclinical evidence for a direct role of MMB in regulating iron homeostasis through the ACVR1/hepcidin axis. Evaluation of the activity of MMB on the ACVR1/hepcidin axis is being done in ongoing MMB clinical studies in patients with MF.
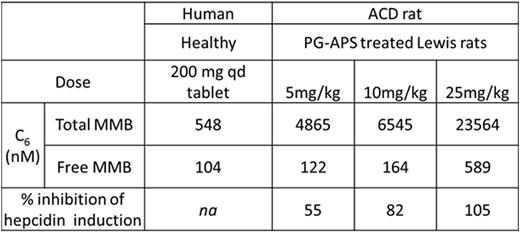
MMB exposure levels and % inhibition of serum hepcidin induction in ACD rats. Human (healthy volunteer) and ACD rat exposure levels for MMB.
MMB exposure levels and % inhibition of serum hepcidin induction in ACD rats. Human (healthy volunteer) and ACD rat exposure levels for MMB.
Tissue distribution of radioactivity in rats following a single oral dose of [14C]-MMB. LOQ is 4, 159 ng-eq/g. 24h rat blood levels were below LOQ.
Tissue distribution of radioactivity in rats following a single oral dose of [14C]-MMB. LOQ is 4, 159 ng-eq/g. 24h rat blood levels were below LOQ.
Warr:Gilead Sciences: Employment, Equity Ownership. Zheng:Gilead Sciences: Employment, Equity Ownership. Sharma:Gilead Sciences: Employment, Equity Ownership. Maciejewski:Gilead Sciences: Employment, Equity Ownership. Theurl:Gilead Sciences: Research Funding. Whitney:Gilead Sciences: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

![Figure 1. Tissue distribution of radioactivity in rats following a single oral dose of [14C]-MMB. LOQ is 4, 159 ng-eq/g. 24h rat blood levels were below LOQ.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/22/10.1182_blood.v128.22.1967.1967/3/m_1967fig02.jpeg?Expires=1763483603&Signature=TL4hqCTJbYTjSdpBYziBS9889hnzU8xgWH6FFExbsgldMqyNoM~tD3nXs2bZq2pnZoP05WH-BnjS1cvZFFFnsMmX-rt5j-6mo5PO1mcSpB8OFfFpQhOfmptLOumTKAgcgwm8eWQc6dV740PrQl-hdECNShBv0J7ZJqas9XxDUFd4K0cyTuUvfKNs8XgX7uKYnj~RTUGj92~nc1FVWjlivQVP0-jAVyZ69pAC0ni6oyDEpx0j2a1Q6al26NEcSc90BMc4HUMU5XKV71s-vSHCG7dLzF3Rg7b~J1p51FUFn9UvSVs~nu4oUrbGmU6Re9QCitx0beQtenymVOt9CeU88Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal