Abstract
Introduction
In Acute Myeloid Leukemia (AML) internal tandem duplications (ITD) in the fms-related tyrosine kinase 3 (FLT3) are a frequent event associated with an unfavorable prognosis. At diagnosis, the FLT3-ITD status is routinely assessed by fragment analysis of PCR-amplified cDNA. However, this assay only provides information on the length but not on the position and sequence of the insertion. Therefore, it is attractive to overcome this limitation by the use of high-throughput amplicon sequencing (HTAS) as an alternative strategy for FLT3-ITD detection. To proof the feasibility and accuracy of this approach we performed HTAS on 260 AML patients, of which 250 were FLT3-ITD positive according to routine diagnostics.
Patients and Methods
All samples were obtained from patients treated on the German Acute Myeloid Leukemia Cooperative Group (AMLCG) trials 1999, 2004 and 2008. All patients received intensive induction therapy with curative intent. At diagnosis, patients had a median age of 60 years (range, 18-80 years). Additional molecular marker were screened routinely: NPM1 mutation (62%), KMT2A-PTD (8%), CEBPA mutations (8%). According to the ELN-classification patients clustered into the following groups: ELN intermediate I (70%), intermediate II (21%) or adverse (9%). A normal karyotype was observed in 71%, while 29% had an aberrant karyotpye. All patient samples were analyzed routinely by FLT3 fragment analysis. For HTAS of FLT3 we used custom FLT3 cDNA primers including barcode and adapter-sequences enabling a one-step PCR-protocol. Sequencing was performed (2x250bp paired end) on a MiSeq (Illumina). Per run, up to 96 samples were sequenced, yielding a median of 79,110 reads per amplicon (range: 31,996 - 259,783). As controls, cDNA from FLT3-ITD positive (Molm-13) and negative (HL60) cell lines were used. Sequencing data were aligned to the FLT3 cDNA reference (NM_004119.2) and FLT3-ITDs were called using Pindel software (version 0.2.5a7).
Results
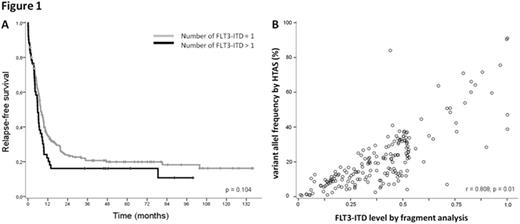
Based on the HTAS results obtained from the FLT3 wild-type control HL60, we set the cut-off for ITD detection at a variant allele frequency (VAF) of 0.5%. Using this threshold, all patients which were assessed to be FLT3-ITD negative in diagnostic routine, were also negative in HTAS. FLT3-ITDs were detected by HTAS in 242 out of 250 (97%) patients who were FLT3-ITD positive patients according to routine fragment analysis. For six out of the 8 remaining patients, no valid ITD was detected by HTAS possibly due to one of the following reasons: a low mutational burden resulting in a VAF below the cut-off level (n=4) or a deletion near the ITD (n=2) potentially interfering with ITD-detection. In total, 308 ITDs were detected in 242 patients by HTAS while in these patients 282 ITDs were detected by routine diagnostics. Of note, HTAS missed 13 subclonal ITDs reported in routine, while 39 additional subclonal ITDs were detected by HTAS only. Overall, HTAS detected a higher number of ITDs per patient (mean: 1.27; range: 1-4) compared to fragment analysis (mean: 1.17; range: 1-3). Patients with more than one ITD according to HTAS showed a trend towards shorter overall and relapse free survival (p=0.105, p=0.104 respectively; Figure 1A). The ITD position (i.e. affected FLT3 domain) based on HTAS did not impact on clinical outcome. There was a significant correlation between the FLT3-ITD levels detected by fragment analysis and HTAS (Pearson, R=0.801, p=0.01; Figure 1B). High FLT3-ITD levels measured by fragment analysis had a significant impact on RFS, whereas this effect was not observed for FLT3-ITD levels measured by HTAS. The quantification of FLT3-ITD by HTAS might be hampered by underestimation of the VAFs of long ITDs that are less likely to be mapped correctly compared to shorter ITDs.
Conclusion
In summary, our study demonstrates the feasibility of HTAS for FLT3-ITD detection in AML. In particular, the identification of subclonal ITDs with high sensitivity provides additional information with potential prognostic value. Thus, HTAS may serve as a robust tool that could be implemented in future diagnostic routines. However, bioinformatic algorithms for ITD detection may need further improvement, e.g. to optimize ITD quantification and to facilitate the detection of ITDs in combination with deletions.
Hiddemann:Roche: Other: Grants; Genentech: Other: Grants; Roche: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal