Abstract
Background
Nucleoside analogues such as cladribine can increase the efficacy of ara-C by modulating deoxycytidine kinase. The addition of cladribine to standard 7+3 chemotherapy has been shown to improve survival in pts with AML (Holowiecki JCO 2012). High dose ara-C (HiDAC) during induction improves outcomes for younger pts (Burnett JCO 2013, Willemze JCO 2013). We conducted a phase-2 clinical trial to study the efficacy of cladribine combined with HiDAC and idarubicin as induction regimen in younger pts with AML.
Methods
The 3-drug treatment protocol consisted of the combination of cladribine, idarubicin, and ara-C (CLIA) in pts with AML aged ≤ 65. Three cohorts were enrolled: de novo AML, secondary AML (s-AML), and relapsed/refractory (salvage). S-AML was defined as pts with AML arising from MDS, MPN, or AA and treated for that disorder prior to enrolling. Induction was cladribine 5 mg/m2 IV over 30 minutes on days 1-5, followed by ara-C 1000 mg/m2 IV on days 1-5, and idarubicin 10 mg/m2 IV days 1-3. Consolidation consisted of up to 5 more cycles of CLIA. Pts with FLT3-ITD could receive sorafenib 400mg PO BID added to CLIA. Pts with FLT3+ disease, presenting WBC count > 100 K/uL, or LDH > 700 IU/dL received prophylactic intrathecal ara-C at count nadir during cycle 1. Mutation profiling was performed using next generation sequencing prior to starting therapy.
Results
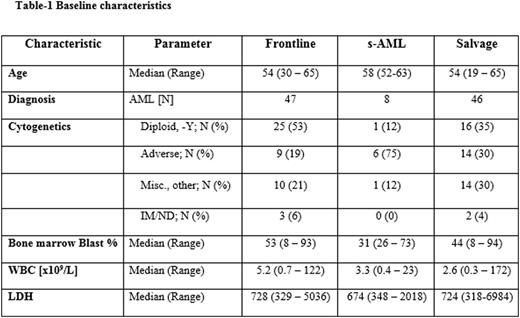
A total of 101 pts were enrolled, with a median age of 55 years (range, 19-65), including 47 pts (47%) in the frontline cohort, 8 (8%) pts in the s-AML cohort, and 46 (46%) in the salvage cohort. Pt characteristics by cohort are outlined in Table 1. In the frontline cohort, 47 pts, who received a median of 3 (1-6) cycles, were evaluable for response. 36 pts achieved CR (77%) after a median of 1 (1-4) cycle and 4 pts CRp, giving an ORR of 81%. The 4- and 8-week mortality rates were 0% and 4%, respectively. In s-AML cohort, 8 pts were evaluable for response, receiving a median of 2 courses (1-3). The rates of CR, CRp, and CRi were 0% (0/8), 25% (2/8), and 0% (0/8) for an ORR of 25%. The 4- and 8-wk mortality rates were 13% and 13%. In the salvage cohort, 46 pts received a median of 1 (1-4) cycle, and were evaluable for response. Ten pts achieved CR (22%), 4 CRp (9%), and 4 CRi (9%), achieving an overall response rate (ORR) of 39%. A median of 1 (1-3) cycle was required for response. The 4- and 8-wk mortality rates were 7% and 15%, respectively. At the time of CR, 21 (55%), 0 (0%), and 8 (44%) of pts achieved MRD negativity by multiparameter flow cytometry in the frontline, s-AML, and salvage cohorts respectively. Pts in the salvage cohort were previously treated with a median of 2 (1-5) prior therapies. ORR by subgroup is summarized in Table 2. After a median follow-up of 12 months (0.9 - 24.1), the 6-month OS estimates were 89%, 26% and 58% and 6 month remission durations were 95%, 100%, and 55% for the frontline, s-AML, and salvage cohorts, respectively (Figure 1A-B). Among MRD negative pts, 1 frontline pt relapsed at 6.7 months and 4 salvage pts relapsed at month 3.4, 3.9, 5.1 and 5.2 (median 4.5). In frontline pts, pretreatment serum ferritin level of < 1000 was associated with improved OS compared to those who had ferritin ≥ 1000 (1-year OS 81% vs. 47%, P=0.04). The regimen was well tolerated. The most common ≥ grade 3 non-hematologic adverse events were fever/infection (17), tumor lysis syndrome (1), cardiac arrhythmia (1), Rash (1), elevated bilirubin (1) and creatinine (1).
Conclusion
The 3-drug combination, CLIA, is safe and effective in younger pts with AML. Outcomes in pts with s-AML were poor, highlighting a subgroup of AML that should be handled separately. Response rates for pts in the newly-diagnosed AML, FLT3-ITD+, and early salvage settings are promising and should be explored further in larger studies and compared to current standard regimens.
Jabbour:Bristol-Myers Squibb: Consultancy. Daver:Kiromic: Research Funding; Pfizer: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; Ariad: Research Funding; Sunesis: Consultancy, Research Funding; BMS: Research Funding; Karyopharm: Honoraria, Research Funding. DiNardo:Novartis: Other: advisory board, Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Abbvie: Research Funding; Agios: Other: advisory board, Research Funding; Celgene: Research Funding. Jain:Genentech: Research Funding; Servier: Consultancy, Honoraria; Novimmune: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Celgene: Research Funding; BMS: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Abbvie: Research Funding; Infinity: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal