Abstract
BACKGROUND
The hypomethylating agents decitabine and 5-azacytidine are commonly used for the initial treatment of acute myeloid leukemia (AML) in elderly patients deemed unfit to receive standard cytotoxic induction chemotherapy. Decitabine at the approved schedule of 20 mg/m2daily for 5 days was shown to be superior to supportive care or low dose cytarabine. (Kantarjian H, JCO; 30(21):2670, 2012). An extended 10-day schedule of decitabine was reported to have a significantly higher response rate (Blum W, PNAS; 107(16):7473, 2010)
OBJECTIVES
The primary objective of the current study was to compare the response rates of the two schedules of decitabine in patients with AML aged 60 years or older who were deemed ineligible to receive standard cytotoxic induction chemotherapy. Secondary objectives were to compare response durations, survival outcomes, and toxicities between the two schedules.
METHODS
Patients with AML aged 60 years or older were randomized using a Bayesian adaptive design to receive decitabine at a dose of 20 mg/m2intravenously daily for either 5 days or 10 days. Responding patients could receive up to 24 cycles repeated every 4 to 8 weeks. Patients on the 10-day schedule could receive 5-day dosing after achieving a response or earlier if clinically indicated. The primary efficacy outcome was complete remission (CR), CR with incomplete count recovery (CRi), or CR with incomplete platelet recovery (CRp) after three cycles.
RESULTS
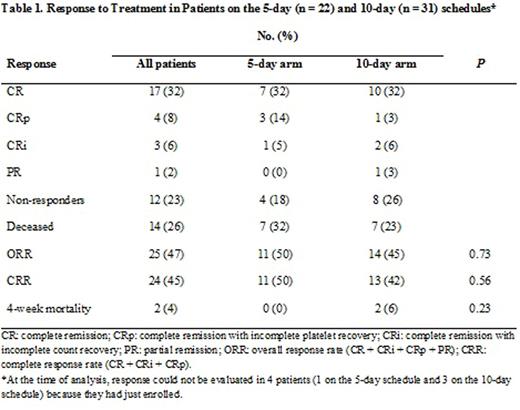
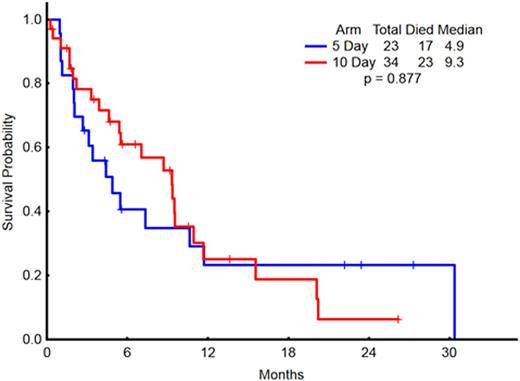
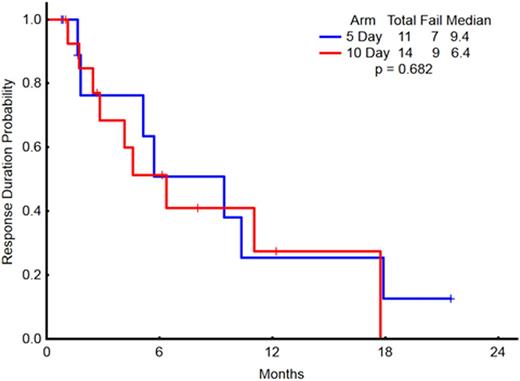
Fifty-seven patients with a median age of 77 years (range 62-90 years) were enrolled on the 5-day (23 patients, 40%) and 10-day (34 patients, 60%) schedules. Thirty-nine patients (68%) were male. The median WBC count in the 5-day and 10-day arms were 2 × 109/L (range 0.7-36.5) and 3 × 109/L (range 0.6-17.7), respectively (p=not significant, NS). The median pre-treatment platelet count for the 5-day and 10-day arms were 24 x 109/L (range 7-162) and 39.5 x 109/L (range 2-213), respectively (p = 0.04) but all other pre-treatment characteristics including the distribution of cytogenetics risk group were similar between the two groups. Pre-treatment cytogenetic findings included complex in 29 patients (51%), diploid in 17 (30%), and intermediate or miscellaneous in 7 (12%), and cytogenetic analysis was not performed in 4 patients (7%). One patient in the 5-day arm received prior treatment for myelodysplastic syndrome and 2 patients in the 10-day arm received prior treatments for chronic myeloid leukemia and post-essential thrombocythemia myelofibrosis (none with hypomethylating agents). Overall and complete response rates did not differ between the two arms (Table 1). Median overall survival was 4.9 months for the 5-day arm and 9.3 months for the 10-day arm (p = 0.88; Figure 1). Median event-free survival was 3.1 months for the 5-day arm and 4.6 months for the 10-day arm (p = 0.92). The median response duration was 9.4 months for the 5-day arm and 6.4 months for the 10-day arm (p = 0.68; Figure 2).
CONCLUSION
Response rates, response durations, and overall survival were not statistically different between the two schedules of decitabine in elderly patients with AML ineligible for cytotoxic induction regimens. These findings should be considered in the design of trials investigating combinations of hypomethylating agents with molecularly targeted agents.
Overall Survival in Patients on the 5-day (n = 23) and 10-day (n = 34) schedules
Overall Survival in Patients on the 5-day (n = 23) and 10-day (n = 34) schedules
Response Duration in Patients achieving CR/CRp/CRi, on the 5-day (n = 11) and 10-day (n = 14) schedules
Response Duration in Patients achieving CR/CRp/CRi, on the 5-day (n = 11) and 10-day (n = 14) schedules
Daver:Kiromic: Research Funding; Karyopharm: Honoraria, Research Funding; Pfizer: Consultancy, Research Funding; BMS: Research Funding; Sunesis: Consultancy, Research Funding; Ariad: Research Funding; Otsuka: Consultancy, Honoraria. DiNardo:Agios: Other: advisory board, Research Funding; Novartis: Other: advisory board, Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Abbvie: Research Funding; Celgene: Research Funding. Wierda:Genentech: Research Funding; Gilead: Research Funding; Acerta: Research Funding; Abbvie: Research Funding; Novartis: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal