Abstract
Background: The combination of 5-azacytidine (AZA) and sorafenib has been reported to be a safe and effective strategy in patients with relapsed and/or refractory FLT3-ITD mutated acute myeloid leukemia (AML). We hypothesized that combining sorafenib with AZA, may be used effectively in older patients with untreated AML whose leukemic cells harbor the mutation.
Methods: Patients were eligible if they had untreated AML with a FLT3-ITD clone detectable by polymerase chain reaction (at least 10% mutation burden), were 60 years of age or older, and had adequate performance status (ECOG ≤ 2) and organ function. The treatment regimen included AZA 75 mg/m2daily for 7 days combined with sorafenib 400 mg twice daily for 28 days. Cycles were repeated approximately every 4 to 5 weeks. Dose adjustments of both agents, and delay of AZA, based on toxicity were allowed.
Results: Overall, 23 patients with untreated AML with a median age of 74 yrs (range, 61-86 yrs) were enrolled. They included 14 (61%) patients with normal cytogenetics, 2 (9%) with complex karyotype, 4 (17%) with other miscellaneous abnormalities, and 3 (13%) with insufficient metaphases. Prior to the initiation of treatment, FLT3-ITD was detected in all patients with a median allelic ratio of 0.35 (range, 0.01-0.89).
The overall response rate in 22 evaluable patients was (77%) including 7 (32%) with CR, 9 (41%) CRi/CRp, and 1 (5%) PR. Patients have received a median of 3 (range, 1-35) treatment cycles with the median number of cycles to response being 2 (range, 1-5) and the median time to achieve response, 1.9 months (range, 0.7-4.3 months). The median duration of CR/CRp/CRi is 14.5 months (range, 1.2-28.7 months). Two (9%) patients have proceeded to allogeneic stem cell transplant.
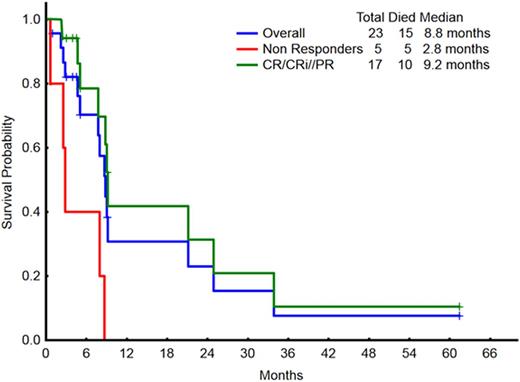
With a median follow-up of 4.2 months (range, 0.9-61.4), 8 patients remain alive, 7 still in remission (CR/CRP/CRi). The median overall survival for the entire group is 8.8 months, and 9.2 months in the 17 responding patients (Figure 1). Treatment-related grade 3/4 adverse events included: grade 3 diarrhea (n=2), grade 3 pneumonitis (n=3), grade 4 sepsis (n=2), grade 3 infections (n=3).
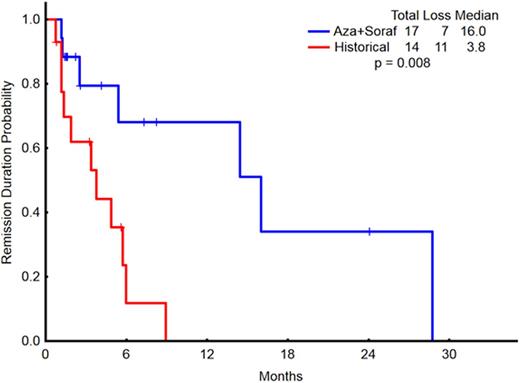
When patients treated with AZA + sorafenib (n=23) were compared to a matched cohort of historical patients older than 60 years who were treated with hypomethylator-based therapy without sorafenib (n=20), overall response rates (including CR, CRp, CRi, and PR) were statistically similar (77% vs.31%, respectively; p=0.6). The median overall survival for the two groups were 8.8 months and 9.4 months (p=0.67), respectively. The remission duration for the responding patients treated with AZA+sorafenib was significantly longer (16 months) than those on other hypomethylator-based regimens without sorafenib (3.8 months)(p=0.008) (Figure 2).
Conclusions: The combination of AZA and Sorafenib is effective and well tolerated in older patients with untreated FLT3-ITD mutated AML.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Daver:Pfizer: Consultancy, Research Funding; Kiromic: Research Funding; BMS: Research Funding; Karyopharm: Honoraria, Research Funding; Otsuka: Consultancy, Honoraria; Sunesis: Consultancy, Research Funding; Ariad: Research Funding. Burger:Roche: Other: Travel, Accommodations, Expenses; Pharmacyclics, LLC, an AbbVie Company: Research Funding; Gilead: Research Funding; Portola: Consultancy; Janssen: Consultancy, Other: Travel, Accommodations, Expenses. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal