Abstract
Background: Outcome of patients with R/R ALL is poor. INO is a CD22 monoclonal antibody bound to a calecheamicin, and has shown single-agent activity in R/R ALL with a response rate of 58% and median survival of 6.3 months. The addition of targeted therapy to effective low-intensity chemotherapy might further improve clinical outcome. The aims of this study are to evaluate response rate, progression-free survival (PFS), overall survival (OS), and to assess the safety of this regimen.
Methods: Patients ≥18 years with R/R ALL were eligible. The chemotherapy was lower intensity than conventional hyper-CVAD and referred to as mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses). Rituximab and intrathecal chemotherapy were given for first 4 courses. INO was given on Day 3 of each of the first 4 courses. Patients received INO at 1.8 mg/m2 for cycle 1 followed by 1.3 mg/m2 for subsequent cycles. After the occurrence of veno-occlusive disease (VOD), the dose of INO was modified to 1.3 mg/m2 for Cycle 1 followed by 1.0 mg/m2 for subsequent cycles starting at the Patient #48.
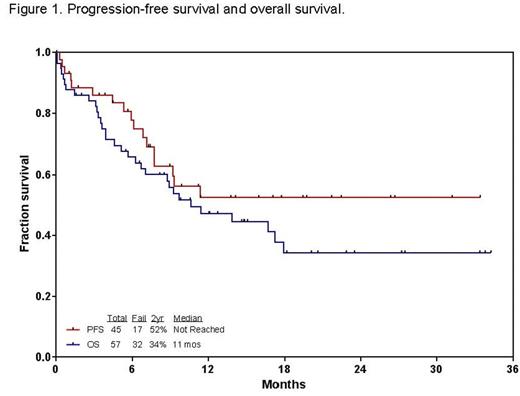
Results: Fifty-seven patients (29 men, 28 women) have been enrolled. Patient characteristics and outcome are summarized in Table 1. Median age is 34 years (range 9-87). Median follow-up is 15 months (range, 2-34). The overall response rate was 45 (79%): 31 (53%) CR, 13 (23%) CR without platelet recovery (CRp), and 1 (2%) CR with incomplete count recovery (CRi). Grade 3/4 toxicities included infections during induction (52%), infections during consolidation (73%), prolonged thrombocytopenia (79%), hyperglycemia (50%), hypokalemia (35%), hyperbilirubinemia (24%), elevated AST/ALT (21%), hemorrhage (18%), and VOD (11%; n=6). Of 6 patients who developed VOD, all 6 had allogeneic stem cell transplantation (allo-SCT) prior to or post INO therapy; three had prior allo-SCT (conditioning regimen; 2 fludarabine/clofarabine; 1 cyclophosphamide/total body irradiation); 4 had allo-SCT after INO therapy (conditioning regimen; 2 fludarabine/busulfan/clofarabine; 1 fludarabine/melphalan; 1 fludarabine/busulfan); 1 had chimeric antigen receptor T-cell therapy. Twenty-seven (47%) patients proceeded to receive allo-SCT. At the last follow-up, 25 (44%) patients are alive. Thirty-two (56%) patients died: 7 early death; 5 refractory disease; 7 post relapse after subsequent salvage, 3 in CR/CRp (1 sepsis; 2 unknown), 10 post allo-SCT (1 VOD; 3 transplant complications; 5 relapse; and 1 unknown). The 2-year PFS and OS rates were 52% and 34%, respectively (Figure 1). Median OS for patients with S1 was not reached with 2-year OS of 53%; patients with S2, 6 months with 2-year OS of 0%; patients with ≥S3, 5.6 months with 2-year OS of 0% (p=0.005). Patients who were treated with mini-hyper-CVD plus INO plus/minus rituximab had a tendency of higher PFS rates, and improved OS compared to a historical cohort with single-agent INO in R/R ALL (2-year PFS; 52% vs. 36%; p=0.20: 2-year OS; 44% vs. 25%; p=0.01).
Conclusions: The combination of INO with low-intensity mini-hyper-CVD chemotherapy is effective and shows encouraging results in patients with R/R ALL. The risk of VOD should be considered carefully for patients with previous liver damage and transplant candidate. Lower dose schedule of INO are being explored.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Burger:Pharmacyclics: Research Funding. Jain:Pharmacyclics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Abbvie: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Seattle Genetics: Research Funding; Genentech: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Servier: Consultancy, Honoraria; Infinity: Research Funding; Celgene: Research Funding; Novimmune: Consultancy, Honoraria. Konopleva:Calithera: Research Funding; Cellectis: Research Funding. DiNardo:Celgene: Research Funding; Agios: Other: advisory board, Research Funding; Abbvie: Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Novartis: Other: advisory board, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal