Abstract
Introduction
Initial treatment with tyrosine kinase inhibitors (TKI) induces excellent response in the majority of patients with CML-CP. Current guidelines recommend periodic monitoring of BCR-ABL1 levels to monitor response. During the course of treatment, recognizing early predictors of deeper response and longer-term outcomes can help guide treatment. This is relevant not only at the specified fixed time point typically reported (i.e., 3, 6, 12 months) but at any other time point when an assessment is made. Achievement of sustained deep molecular response is a goal of increasing relevance as it opens the possibility of treatment discontinuation. The objective of this study is suggest optimal BCR-ABL transcript levels at any given time, and to suggest a prediction model for sustained molecular response 4.5 (MR4.5) (BCR-ABL ≤0.0032%) for at least 2 years according to BCR-ABL levels achieved within the first 12 months of TKI therapy.
Methods
Response data for 630 patients with newly diagnosed CML-CP in consecutive prospective clinical trials of frontline imatinib (n=73; NCT00048672), high-dose imatinib (n=208; NCT00038469 and NCT00050531), nilotinib (n=148; NCT00129740), dasatinib (n=150; NCT00254423), and ponatinib (n=51; NCT01570868) were analyzed. Real-time PCR analysis was performed at approximately 3 month intervals during the first year and 6 month intervals thereafter. The "best fit average" molecular response was defined by robust linear regression models, with which the estimated molecular level in patients with complete cytogenetic response (CCyR) within 1 year, major molecular response (MMR) within 1 year, and sustained MR4.5 at any point were defined. The acceptable molecular response was defined by quantile regression for the 95th percentile, with which the worst 5% BCR-ABL levels in patients with CCyR within 1 year, MMR within 1 year, and sustained MR4.5 at any point were identified.
Results
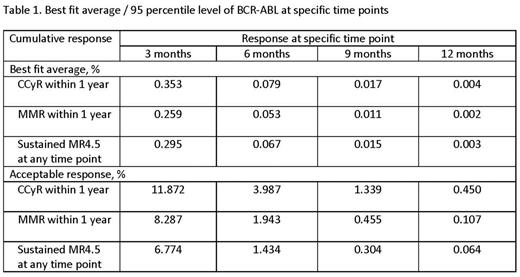
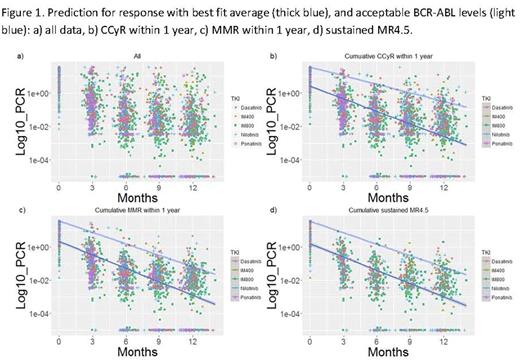
In 630 patients, 2512 data points of BCR-ABL levels within 1 year of TKI were identified. The median follow-up for the entire cohort was 106 months (range, 0.3-177.8). The regression equations for best fit average PCR for CCyR within 1 year was Log10(PCR) = -0.2159 x (Months) + 0.1957; for MMR within 1 year, Log10(PCR) = -0.2304 x (Months) + 0.1046; for sustained MR4.5 at any point, Log10(PCR) = -0.2154 x Months -0.1161. The regression equations for acceptable PCR for CCyR within 1 year was Log10(PCR) = -0.15796 x (Months) + 1.54839; for MMR within 1 year, Log10(PCR) = -0.20999 x (Months) + 1.54839; for sustained MR4.5, Log10(PCR) = -0.22476 x (Months) + 1.50516 (Figure 1). The best fit average PCR (i.e., estimated levels achieved by the average responder in each category) for CCyR within 1 year was 0.353%, 0.079%, 0.017%, and 0.004% at 3, 6, 9, and 12 months, respectively; for MMR within 1 year was 0.259%, 0.053%, 0.011%, and 0.002% at 3, 6, 9, and 12 months, respectively; for sustained MR4.5 at any point was 0.295%, 0.067%, 0.015%, and 0.003% at 3, 6, 9, and 12 months, respectively (Table 1). To achieve CCyR within 1 year, the acceptable PCR (i.e., levels achieved by 95% of all those who eventually reach the said endpoint) response was 11.872%, 3.987%, 1.339%, and 0.450% at 3, 6, 9, and 12 months, respectively; to achieve MMR within 1 year, 8.287%, 1.943%, 0.455%, and 0.107% at 3, 6, 9, and 12 months, respectively; to achieve sustained MR4.5 at any time, 6.774%, 1.434%, 0.304%, and 0.064% at 3, 6, 9, and 12 months, respectively. Of 289 patients who eventually achieved sustained MR4.5, 288 (99%) achieved CCyR within 1 year; 268 (93%), MMR within 1 year; 201 (70%), MR4 within 1 year; 162 (56%), MR4.5 within 1 year; 72 (25%), CMR within 1 year. Of 359 patients who achieved MMR within 1 year with a minimum follow-up of 48 months, 256 (71%) achieved sustained MR4.5; of 180 patients who achieved MR4.5 within 1 year, 151 (84%); of 72 patients who achieved CMR within 1 year, 65 (90%).
Conclusion
Proper interpretation of early transcript levels at any time during the course of therapy may help predict later response and outcome. Such models can be built to guide therapy for patients in a continuous basis. To achieve sustained MR4.5 for at least 2 years, deeper responses are required at each time point. Our model proposes optimal values that predict the highest probability of reaching such goal. At a minimum, CCyR within 1 year is required to achieve sustained MR4.5.
Kantarjian:Bristol-Myers Squibb: Research Funding; ARIAD: Research Funding; Amgen: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Ravandi:Seattle Genetics: Consultancy, Honoraria, Research Funding; BMS: Research Funding. Konopleva:AbbVie: Research Funding; Genentech: Research Funding. Wierda:Novartis: Research Funding; Abbvie: Research Funding; Acerta: Research Funding; Gilead: Research Funding; Genentech: Research Funding. Daver:Ariad: Research Funding; Karyopharm: Honoraria, Research Funding; Sunesis: Consultancy, Research Funding; BMS: Research Funding; Kiromic: Research Funding; Otsuka: Consultancy, Honoraria; Pfizer: Consultancy, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal