Abstract
Current autologous stem cell transplantation and gene therapy strategies are limited by the inability to identify a true hematopoietic stem cell (HSC) propulation that reliably predicts engraftment. The ability to enrich and target a specific HSC pool and predict engraftment levels of the gene-modified cells would be a major advance, reducing manufacturing costs and off-target effects. Here we describe a distinct HSC phenotype, conserved between humans and nonhuman primates (NHP) which overcomes this limitation.
We used our NHP stem cell transplantation and gene therapy model to study the engraftment potential of phenotypically distinct hematopoietic stem and progenitor cell (HSPC) subpopulations. We were able to identify an exclusive HSPC subpopulation capable of multi-lineage engraftment (Figure 1A and 1B). This HSPC subpopulation (denoted "I") accounts for ~3-5% of the entire CD34+ cell population in primed bone marrow, reducing the number of cells targeted for cell and gene therapy approaches by 20 to 30-fold. For autologous transplants, only 300-400K HSPCs/kg body weight were required to achieve rapid neutrophil and plateletet recovery within 9-10 and 19-20 days, respectively. Stable 30-35% gene-marking was obeserved in all blood lineages including T cell, B cell, NK cells, granulocyte, monocytes/macrophages, erythrocytes and platelets. Complete reconstitution of the bone marrow compartment was achieved within <100 days, with 31-33% gene-marking in HSCs as well as downstream progenitors from all lineages.
Most importantly, and in support of the above described engraftment studies, the use of this HSPC population I phenotype allowed us to establish a reliable flow cytometry based analysis to assess and predict engraftment after HSC transplantion. This method predicted the onset of neutrophil and platelet recovery (Figure 1C and 1D). This assay was also able to predict the onset of NHP hematopoietic recovery for transplanted HSCs from different stem cell sources including steady state (unprimed) bone marrow, as well as gene-modified/transduced and ex vivo expanded HSCs. From this data, we were able to define the minimum cell number of HSCs/kg body weight required for sustained multi-lineage long-term engraftment with recovery of all blood cell lineages in the NHP transplant setting as 110,000 cells/kg. Importantly, we demonstrated that this cell population and phenotype is conserved between NHP and human hematopoiesis (Radtke et al. 2016, submitted).
In summary, we identified a distinct and evolutionarily conserved HSC phenotype that is associated with multi-lineage engraftment in nonhuman primates and also reliably predicted the engraftment kinetics after transplant. This will greatly facilitate evaluation and "potency" of autolgous stem cell products especially after stem cell expansion or gene therapy. In addition, it will allow for an improved enrichment of the target cells for gene therapy and gene editing protocols and thus likely improve efficacy and safety.
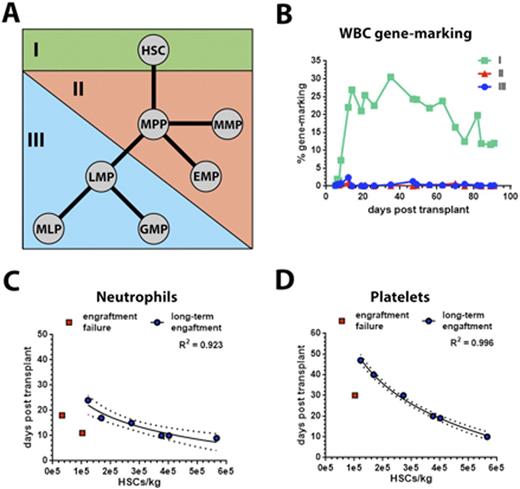
HSCs are exclusively driving multi-lineage engraftment and allow prediction of neutrophil and platelet engraftment in the NHP transplant model.
(A) Hierarchical organization of HSPCs in the NHP. HSPC of group I, II, and III were sort-purified and transduced with LV vectors expressing either green fluorescent protein (GFP; population I), mCherry (population II) or mCerulean (population III), respectively. (B) Long-term follow-up of gene modified white blood cell (WBC) levels in vivo after myeloablative transplantation of these cell populations. (C and D) Correlation of transplanted HSCs/kg body weight with (C) neutrophil and (D) platelet recovery. Animals demonstrating engraftment failure (red squares) were excluded from this correlation.
HSCs are exclusively driving multi-lineage engraftment and allow prediction of neutrophil and platelet engraftment in the NHP transplant model.
(A) Hierarchical organization of HSPCs in the NHP. HSPC of group I, II, and III were sort-purified and transduced with LV vectors expressing either green fluorescent protein (GFP; population I), mCherry (population II) or mCerulean (population III), respectively. (B) Long-term follow-up of gene modified white blood cell (WBC) levels in vivo after myeloablative transplantation of these cell populations. (C and D) Correlation of transplanted HSCs/kg body weight with (C) neutrophil and (D) platelet recovery. Animals demonstrating engraftment failure (red squares) were excluded from this correlation.
Adair:Rocket Pharmaceuticals: Consultancy, Equity Ownership. Kiem:Rocket Pharmaceuticals: Consultancy, Equity Ownership, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal