Key Points
Direct-acting antiviral agents are able to induce lymphoma response in patients with HCV-associated indolent non-Hodgkin lymphoma.
The highest rate of lymphoma response (73%) was observed in patients with marginal zone lymphoma.
Abstract
Regression of hepatitis C virus (HCV)–associated lymphoma with interferon (IFN)-based antiviral treatment supports an etiological link between lymphoma and HCV infection. In addition, a favorable impact of antiviral treatment on overall survival of patients with HCV-related lymphoma has been reported. Data on IFN-free regimens combining direct-acting antivirals (DAAs) in HCV-associated lymphoproliferative disorders are scanty. We analyzed the virological and lymphoproliferative disease response (LDR) of 46 patients with indolent B-cell non-Hodgkin lymphomas (NHLs) or chronic lymphocytic leukemia (CLL) and chronic HCV infection treated with DAAs. The histological distribution was 37 marginal zone lymphomas (MZLs), 2 lymphoplasmacytic lymphomas, 2 follicular lymphomas, 4 CLL/small lymphocytic lymphomas (CLL/SLLs), and 1 low-grade NHL not otherwise specified. Thirty-nine patients received a sofosbuvir-based regimen and 7 patients received other DAAs. The median duration of DAA therapy was 12 weeks (range, 6-24 weeks). A sustained virological response at week 12 after finishing DAAs was obtained in 45 patients (98%); the overall LDR rate was 67%, including 12 patients (26%) who achieved a complete response. The LDR rate was 73% among patients with MZL, whereas no response was observed in CLL/SLL patients. Seven patients cleared cryoglobulins out of 15 who were initially positive. After a median follow-up of 8 months, 1-year progression-free and overall survival rates were 75% (95% confidence interval [CI], 51-88] and 98% [95% CI, 86-100], respectively. DAA therapy induces a high LDR rate in HCV-associated indolent lymphomas. These data provide a strong rationale for prospective trials with DAAs in this setting.
Introduction
In addition to liver disease, hepatitis C virus (HCV) infection has been linked to the development of type II mixed cryoglobulinemia and to a spectrum of B-cell lymphoproliferative disorders.1 Systematic reviews of epidemiological studies evaluating the prevalence of HCV infection in B-cell non-Hodgkin lymphomas (B-NHLs) confirmed that HCV prevalence is higher in patients with B-NHL than in the general population, supporting a role of HCV in the etiology of B-NHL.2 In subtype-specific analysis, HCV infection is associated with marginal zone lymphoma (MZL), diffuse large B-cell lymphoma (DLBCL), and lymphoplasmacytic lymphoma.3 The pathophysiology of HCV-associated B-NHL, at least for MZL, appears to be linked to chronic antigenic stimulation, because several reports show that the clearance of HCV infection with interferon (IFN)-based antiviral therapy often results in regression of the tumor burden.4,5 The favorable impact of IFN-based antiviral therapy on the outcome of these patients has been consistently reported in the Fondazione Italiana Linfomi study5 and in The French Research Agency ANRS (France REcherche Nord&Sud Sida-HIV Hépatites) HC-13 Lympho-C study.6 However, a direct antiproliferative effect of IFN on malignant lymphocytes cannot be ruled out.
The therapy of HCV infection is undergoing a transformation. After nearly 25 years of IFN-based therapies, a new era for direct-acting antiviral (DAA) drugs has entered clinical practice.7,8 Recently, several DAAs were approved as part of different IFN-free combination therapies. These include second-generation inhibitors of nonstructural (NS) protein NS3/4A (simeprevir, ritonavir-boosted paritaprevir, and grazoprevir), NS5A inhibitors (daclatasvir, ledipasvir, ombitasvir, and elbasvir), a nucleotide polymerase inhibitor (sofosbuvir), and a non-nucleoside polymerase inhibitor (dasabuvir). In terms of efficacy, DAA therapy can induce viral eradication in >90% of all patients across different genotypes and fibrosis stages, with the exception of some patient subgroups (eg, patients with decompensated cirrhosis).9 Furthermore, DAA regimens are associated with a better safety and tolerability profile than IFN treatment and, thus, may represent an attractive alternative to manage HCV-associated NHL simultaneously.
However, data on the efficacy of IFN-free regimens10 in HCV-associated lymphoproliferative disorders are scanty and based on clinical reports.11,12 In this study, we have evaluated the virological and lymphoproliferative disease response (LDR) rates and toxicity of DAAs in a large series of patients with indolent lymphoproliferative disorders treated by DAAs in the absence of immunochemotherapy.
Methods
Study design and patients
We retrospectively selected patients affected by indolent B-NHL or chronic lymphocytic leukemia (CLL) and infected by HCV, defined as HCV-RNA positivity, and who were treated with DAAs within Italian centers of Fondazione Italiana Linfomi, in the French ANRS-CO22 HEPATHER cohort, in a German center (Frankfurt), and in a US center (Houston, TX). Five patients were already reported in the literature10-12 and were included with updated follow-up. Approval for this study, which was based on the use of archival or cohort data, was obtained from the Institutional Review Boards of the participating centers. The report was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology Statement.13 Data management and analysis were carried out in accordance with the tenets of the Declaration of Helsinki of 1964, as revised in 2000. All patients gave informed consent.
Clinical and virological end points
The primary end point was overall LDR rate; complete response (CR), partial response (PR), progression-free survival (PFS), and overall survival (OS) rates were secondary end points. PFS was defined as the time interval between the start of DAA therapy and lymphoma progression, initiation of new treatment, or death. Response evaluation was based on Lugano classification criteria for lymphomas14 and on guidelines from the International Workshop on CLL.15 Specific examinations were carried out for response assessment in specific lymphoma subtypes according to clinical presentation (ie, paraprotein level when present before DAA). Bone marrow biopsy was repeated at the end of DAA only in cases that were positive at study entry. Response assessment was performed 1 month after the end of DAA therapy. Sustained virological response was defined as undetectable HCV viral load (<15 IU/mL) 12 weeks after completion of therapy.
Statistical analysis
Fisher’s exact test was used to explore associations between categorical variables. The Wilcoxon rank-sum test was applied to test differences of quantitative variables between two independent groups. The associations between clinical variables and nonresponse were reported in terms of odds ratio (OR), 95% confidence interval (CI), and significance level (P value). All variables with a P value ≤.1 in the univariate analysis of nonresponse were included in a multivariate logistic model. The Kaplan-Meier product-limit method was used to estimate PFS and OS. All computations were carried out using Stata 12.1 (2007).
Results
Hematological and virological features
Hematological and virological characteristics of the 46 study patients are summarized in Table 1. There was a slight female predominance (61%), and median age was 59 years. Only 3 patients had CLL, whereas the other 43 patients had NHL. The main histological NHL type was MZL (37 patients, 81%), among which 15 were extranodal MZL of mucosa-associated lymphoid tissue (all extragastric and 3 with multiple mucosa-associated lymphoid tissue sites). Among all NHL patients, the predominantly involved sites were spleen (n = 22) and liver (n = 6). A serum monoclonal component was present in 19 patients, and cryoglobulins were detectable in 15 patients, among which 6 were symptomatic.
Features of the 46 patients with B-cell lymphoproliferative disorders associated with HCV infection treated with DAAs
| . | n . | % . |
|---|---|---|
| Male/female | 18/28 | 39/61 |
| MZLs | 37 | 80 |
| Splenic | 17 | 37 |
| Nodal | 1 | 2 |
| Extranodal | 15 | 32 |
| Leukemic | 4 | 9 |
| Others* | 5 | 11 |
| CLL/SLL | 4 | 9 |
| Ann Arbor stage III-IV | 35/42 | 83 |
| B symptoms | 6 | 13 |
| ECOG performance status ≥2 | 1 | 2 |
| Hemoglobin <12 g/dL | 14/45 | 31 |
| Platelets <100 × 109/L | 10/45 | 22 |
| Lactate hydrogenase > UNL | 10/40 | 25 |
| β2-Microglobulin > UNL | 20/26 | 77 |
| Albumin <3.5 g/dL | 6/40 | 15 |
| HCV genotype | ||
| 1 | 29 | 63 |
| 2 | 12 | 26 |
| 3 | 3 | 7 |
| 4 | 2 | 4 |
| Cirrhosis | 7 | 15 |
| Previous chemotherapy | 10 | 22 |
| Previous IFN-based antiviral treatment | 12 | 26 |
| DAAs | ||
| Sofosbuvir-based regimen† | 39 | 85 |
| Other regimen‡ | 7 | 15 |
| . | n . | % . |
|---|---|---|
| Male/female | 18/28 | 39/61 |
| MZLs | 37 | 80 |
| Splenic | 17 | 37 |
| Nodal | 1 | 2 |
| Extranodal | 15 | 32 |
| Leukemic | 4 | 9 |
| Others* | 5 | 11 |
| CLL/SLL | 4 | 9 |
| Ann Arbor stage III-IV | 35/42 | 83 |
| B symptoms | 6 | 13 |
| ECOG performance status ≥2 | 1 | 2 |
| Hemoglobin <12 g/dL | 14/45 | 31 |
| Platelets <100 × 109/L | 10/45 | 22 |
| Lactate hydrogenase > UNL | 10/40 | 25 |
| β2-Microglobulin > UNL | 20/26 | 77 |
| Albumin <3.5 g/dL | 6/40 | 15 |
| HCV genotype | ||
| 1 | 29 | 63 |
| 2 | 12 | 26 |
| 3 | 3 | 7 |
| 4 | 2 | 4 |
| Cirrhosis | 7 | 15 |
| Previous chemotherapy | 10 | 22 |
| Previous IFN-based antiviral treatment | 12 | 26 |
| DAAs | ||
| Sofosbuvir-based regimen† | 39 | 85 |
| Other regimen‡ | 7 | 15 |
Median age of patients was 59 years (range, 40-78 years).
ECOG, Eastern Cooperative Oncology Group; UNL, upper normal limit.
Follicular lymphoma (n = 2), lymphoplasmacytic lymphoma (n = 2), and low-grade B-NHL not otherwise specified (n = 1).
Sofosbuvir combined with simeprevir (n = 13), ribavirin (n = 15), daclatasvir (n = 8), or ledipasvir (n = 3).
Paritaprevir/ritonavir/ombitasvir with or without dasabuvir with or without ribavirin (n = 6) or faldaprevir/deleobuvir/ribavirin (n = 1).
Seven patients were cirrhotic (5 Child-Pugh class A and 2 Child-Pugh class C). No patient was co-infected with HIV, and 1 patient had concomitant hepatitis B virus infection (hepatitis B virus surface antigen positive). Ten patients had previously received chemotherapy, and 12 had received an IFN-based antiviral regimen. Treatment was a sofosbuvir-based regimen in all but 7 patients. Median duration of treatment was 12 weeks (range, 6-24 weeks). In 1 patient with renal MZL, 4 weekly rituximab doses were added to DAA. All patients, except 1 who received only 6 weeks of DAA therapy for early progression, were treated with the whole course of DAA therapy.
Toxicity
Toxicity of treatment was negligible: 13 patients had grade 1 to 2 adverse events (including 5 cases of anemia in patients treated with ribavirin), and only 1 grade 3 event was registered (asthenia).
Virological response and LDR
DAA treatment led to a virological response in all patients, except 1 who had decompensated cirrhosis. Hematological ORR was 67%, CR was obtained in 12 cases (26%), and PR was obtained in 19 (41%). Eleven patients had stable disease (24%) and four early progressed. The LDR rate was 73% in patients with virological response and absent in the single case without virological response. Responses according to histological subtypes are summarized in Table 2. The ORR was 73% in MZL (27/37) and 44% in non-MZL lymphoproliferative disorders (4/9). Remarkably, none of the 4 CLL/SLL cases exhibited responses. Among 7 cirrhotic patients, 1 obtained a PR, 4 exhibited disease, and 2 exhibited progressive disease, whereas 6 had a virological response. Lastly, 7 patients out of 15 who were initially positive, cleared cryoglobulins.
LDR to DAAs according to histological subtypes in 46 patients with B-cell lymphoproliferative disorders associated with HCV infection
| . | CR, n . | PR, n . | SD, n . |
|---|---|---|---|
| All (N = 46) | 12 | 19 | 11 |
| MZLs (n = 37) | 11 | 16 | 6 |
| Splenic (n = 17) | 4 | 7 | 5 |
| Nodal (n = 1) | 1 | 0 | 0 |
| Extranodal (n = 15) | 5 | 7 | 0 |
| Leukemic (n = 4) | 1 | 2 | 1 |
| Follicular lymphoma (n = 2) | 0 | 2 | 0 |
| Lymphoplasmacytic lymphoma (n = 2) | 0 | 1 | 1 |
| Low-grade B-NHL NOS (n = 1) | 1 | 0 | 0 |
| CLL/SLL (n = 4) | 0 | 0 | 4 |
| . | CR, n . | PR, n . | SD, n . |
|---|---|---|---|
| All (N = 46) | 12 | 19 | 11 |
| MZLs (n = 37) | 11 | 16 | 6 |
| Splenic (n = 17) | 4 | 7 | 5 |
| Nodal (n = 1) | 1 | 0 | 0 |
| Extranodal (n = 15) | 5 | 7 | 0 |
| Leukemic (n = 4) | 1 | 2 | 1 |
| Follicular lymphoma (n = 2) | 0 | 2 | 0 |
| Lymphoplasmacytic lymphoma (n = 2) | 0 | 1 | 1 |
| Low-grade B-NHL NOS (n = 1) | 1 | 0 | 0 |
| CLL/SLL (n = 4) | 0 | 0 | 4 |
NOS, not otherwise specified; SD, stable disease.
Because no LDR was observed among the CLL/SLL patients, we analyzed the predictive factors of LDR within the 42 cases of NHL only. In univariate analysis, there was a trend toward a higher risk of nonresponse in patients with nodal disease and in patients with low hemoglobin levels. In contrast, patients with extranodal disease and those with a serum monoclonal component have shown a trend toward a lower risk of nonresponse (P < .1) (see supplemental Table 1, available on the Blood Web site). In multivariate analysis, risk of nonresponse was significantly lower in patients with a serum monoclonal component compared to those without (OR, 0.1; 95% CI, 0.1-1.0; P = .048). Furthermore, the presence of extranodal disease showed a trend toward a lower risk of nonresponse (OR, 0.1; 95% CI, 0.1-1.1; P = .059). On the contrary, no effect was found for the presence of nodal disease (OR, 6.5; 95% CI, 0.5-81.9; P = .150) or for low hemoglobin level (OR, 2.4; 95% CI, 0.4-16.4; P = .357).
Outcome
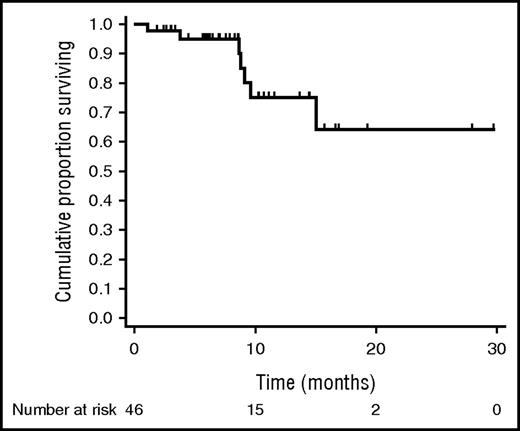
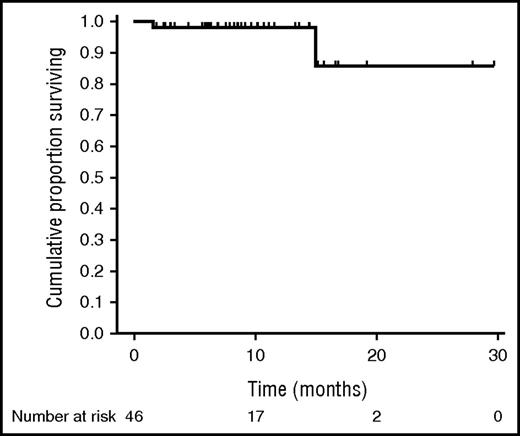
After a median follow-up of 8 months (range, 2-30 months) since the start of DAA therapy, median PFS is not reached, and estimated 1-year PFS is 75% (95% CI, 51-88) (Figure 1). Median OS is not reached, and estimated 1-year OS is 98% (95% CI, 86-100) (Figure 2). Four early progressions occurred either during (n = 1) or within 3 months after the end of DAA therapy (n = 3). The first patient was treated with DAA because of Child class C cirrhosis with encephalopathy and concomitant extranodal MZL with high tumor burden; he had no virological or lymphoma response and died 4 weeks after the early interruption of DAA therapy. Another extranodal MZL patient relapsed soon after the interruption of DAA and received immunochemotherapy that led to CR. One patient with splenic MZL (SMZL), despite a rapid virological response, developed a rapidly growing kidney mass that was diagnosed as transformed DLBCL. One patient with a previous diagnosis of extranodal MZL had an early progression after DAA therapy and is now under chemotherapy treatment.
PFS of 46 patients with B-cell lymphoproliferative disorders associated with HCV infection treated with DAA agents.
PFS of 46 patients with B-cell lymphoproliferative disorders associated with HCV infection treated with DAA agents.
OS of 46 patients with B-cell lymphoproliferative disorders associated with HCV infection treated with DAA agents.
OS of 46 patients with B-cell lymphoproliferative disorders associated with HCV infection treated with DAA agents.
Two patients with SMZL progressed more than 3 months after the end of DAA therapy. One was treated with immunochemotherapy, resulting in PR. One patient progressed 6 months after the end of DAA therapy with worsening cytopenias but is still in observation. Lastly, a patient with SMZL died of hepatocellular carcinoma 8 months after the end of DAA therapy while in hematological PR (8 months after the end of DAA therapy).
Discussion
Here, we report the first large series of patients with HCV-associated lymphoproliferative disorders treated with IFN-free DAA therapy. As described in HCV infection without lymphoma,16 this study confirms that virological response is obtained in nearly all patients with chronic HCV infection (98%). This high rate of virological response was associated with an overall LDR rate of 67%, including CR and PR in 26% and 41% of patients, respectively.
Epidemiological studies provide strong evidence for an association of HCV infection with B-cell lymphoproliferative disorders, in particular, with the histological subtypes MZL17 and DLBCL.18,19 The most convincing evidence for a causal relationship between HCV infection and lymphoma development is the observation of lymphoma regression after HCV eradication by antiviral therapy.4-6,20 In a recent meta-analysis of 20 studies (254 patients) evaluating the efficacy of IFN-based treatment in HCV-related NHL, overall lymphoma response rate after IFN-based antiviral therapy was 73%, which is similar to the ORR reported in the present study.21 In studies using IFN-based antivirals, in which the rate of virological response was lower, a strong correlation between virus clearance and lymphoma response has been established. In the present study, the only patient who did not achieve sustained virological response to DAA at week 12 also underwent lymphoma progression leading to early death.
Regarding the association between histological type and lymphoma response, in the above-cited meta-analysis,21 there was a trend toward favorable response for antivirals in HCV-associated MZL (response rate, 81%), compared with non-MZLs (response rate, 71%). The higher lymphoma response rate in MZL is confirmed in our series: the ORR is 73% for MZL and 44% for non-MZL. Of note, the MZL subtype exhibits similar response rates to those obtained with an IFN-based regimen. Taking into account the relatively low number of cases, we found trends for a better lymphoma response in patients with extranodal disease and paraproteinemia, features typically associated with HCV-associated NHLs.22-24 Lastly, for CLL/SLL, which is frequently not epidemiologically associated with HCV infection,3,25 no LDRs were achieved despite virus clearance. Altogether, these findings confirm that HCV clearance per se is beneficial in patients with non-CLL/SLL HCV-associated B-NHL and support a stepwise model of lymphomagenesis induced by chronic antigenic stimulation in this setting.
Considering the favorable impact of IFN-based antivirals on the outcome of HCV-infected NHL patients,5,6 our data strongly suggest that antiviral treatment should be used as the first option for HCV-associated MZL when cytoreductive treatment is not immediately necessary.26-28 An extended follow-up of this series will be necessary to confirm that responses are sustainable, even in the group of patients in PR as long as virological response is maintained, suggesting that hematological CR is not a mandatory therapeutic goal to reach in this setting. Although follow-up is still short, especially for a series of indolent lymphomas, the rapid lymphoma and virological responses, along with the good safety profile that we describe, suggest that DAA therapy should be used in first-line therapy in patients with HCV-associated MZL, as well as in those with absent or mild liver impairment. Consistent with this recommendation, Torres and Mahale recently demonstrated that the majority of patients with HCV-NHL have low liver fibrosis at NHL diagnosis.29 Our data also support the use of DAA in patients with indolent lymphoma in progression or relapse after previous treatment with immunochemotherapy. Of note, the combination of rituximab and IFN-based antivirals proved to be feasible and efficient in patients with cryoglobulinemia and lymphoproliferative B-cell disorders.30,31 Further studies are warranted to evaluate the place of this combination in the treatment of patients with advanced, rapidly progressive, and/or symptomatic disease.
The limits of this retrospective study are the heterogeneity of the antiviral treatments, the variable length of follow-up, a possible selection bias, and the limited length of follow-up. In particular, due to the retrospective nature of this analysis, the criteria for initiating treatment are probably heterogeneous.
In conclusion, HCV-infected patients with indolent B-NHL, especially of marginal zone origin, benefit from antiviral therapy. Because of DAAs’ safety, rapidity, and efficacy in obtaining a virological response, as well as good tolerance profile, DAA therapy should be preferred to IFN-based antiviral treatment. It can be proposed as first-line therapy in patients in progression or relapse with no need for immediate immunochemotherapy. These results provide a strong rational background for a larger prospective series to determine precisely the impact of DAA therapy in HCV-infected patients with B-NHL.
Presented in abstract form at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 8 December 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The ANRS-CO22 HEPATHER cohort was sponsored and funded by The French Research Agency ANRS (France REcherche Nord&Sud Sida-HIV Hépatites).
The sponsor has not participated in the statistical analysis and interpretation of data, in the writing the manuscript, and in the decision to submit the work for publication.
Authorship
Contribution: L. Arcaini, C.B., C.V., and O.H. designed and supervised the overall conduction of the study; V.V.F., L. Arcaini, M.F., and C.V. analyzed the data; L. Arcaini wrote the manuscript; L. Arcaini and C.V. collected the data; L. Arcaini, C.B., M.F., M.G., M.C., M.V., H.A.T., V.L.-R., J.P.-O., P.F., R.R., F.Z., L.R., S.R., R.B., M.M., H.F., L. Alric, A.J., C.D., S.P., F.C., C.V., and O.H. enrolled and cared for the patients; L. Arcaini, C.B., C.V., and O.H. reviewed the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: L. Arcaini received advisory honoraria from Bayer, Celgene, Gilead Sciences, Roche, and Sandoz and research support from Gilead Sciences. H.A.T is or has been the principal investigator for research grants from Gilead Sciences, Merck & Co., and Vertex Pharmaceuticals, with all funds paid to MD Anderson. H.A.T also is or has been a paid scientific advisor for Gilead Sciences, Janssen Pharmaceuticals, Merck & Co., Vertex Pharmaceuticals, Genentech, Novartis, Astellas Pharma, Pfizer, and Theravance Biopharma; the terms of these arrangements are being managed by MD Anderson Cancer Center in accordance with its conflict-of-interest policies. V.L.-R. received advisory honoraria from Bristol-Myers Squibb (BMS), Gilead Sciences, AbbVie, and Merck Sharp & Dohme (MSD) and research support from BMS and Roche. R.B. received advisory and/or lecture honoraria from AbbVie, BMS, Gilead Sciences, MSD, and Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Luca Arcaini, Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Viale Golgi 19, 27100 Pavia, Italy; e-mail: luca.arcaini@unipv.it.
References
Author notes
L. Arcaini and C.B. contributed equally to this study.
C.V. and O.H. contributed equally to this study.