To the editor:
Acute myeloid leukemia (AML) is considered a sporadic disease caused by sequential accumulation of somatically acquired mutations in hematopoietic stem or progenitor cells (HSPCs). However, familial clustering of myeloid neoplasms is being increasingly observed and attributed to highly penetrant germ line variants in several different genes.1 The recently published “2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia” incorporated these findings and defined “Myeloid neoplasms with germ line predisposition” as a distinct disease entity.2 In addition to a series of developmental syndromes, this group currently comprises cases with germ line mutations in CEBPA, DDX41, RUNX1, ANKRD26, ETV6, and GATA2. Here, we describe familial clustering of AML in a TP53 mutated Li-Fraumeni syndrome (LFS) pedigree. Further screening of 186 primary AML specimens revealed the presence TP53 germ line mutations in 1.1% of cases. Finally, we report their frequent occurrence in therapy-related AML (tAML) arising after antecedent ionizing radiation, an observation of relevance for future studies within this area. The study was approved by the ethical committee of the Medical University of Graz, Graz, Austria (vote numbers 21-065 ex 09/10 and 26-369 ex 13/14, respectively) and performed in accordance with the Declaration of Helsinki.
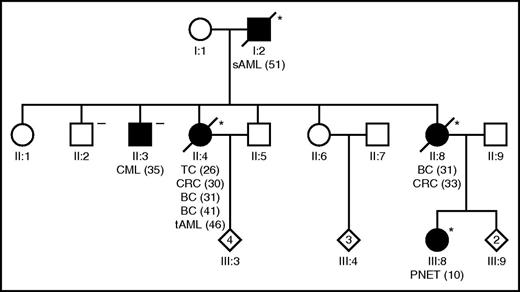
A 46-year-old white woman presented with tAML with a complex karyotype arising after previous administration of chemo- and radiotherapy for papillary thyroid carcinoma at the age of 26, colorectal cancer at the age of 30, and bilateral breast cancer at the age of 31 and 41 years, respectively. Despite 3 lines of intensive AML induction/salvage therapy, the patient never achieved remission and died of progressive disease 7 months after diagnosis. Personal and family history indicated an LFS according to Chompret criteria3 (Figure 1). Interestingly, in addition to the characteristic LFS tumor spectrum, secondary AML following myelodysplasia was observed in the index patient’s father and chronic myeloid leukemia was observed in one of her brothers. Analysis of the TP53 gene was performed using skin fibroblasts and peripheral blood, respectively, as previously described.4,5 A TP53 c.467G>C germ line mutation characterized this LFS pedigree affecting both patients with AML but not the subject suffering from CML (Figure 1; supplemental Table 2, available on the Blood Web site). Although the infrequent occurrence of myeloid neoplasms in individual patients with LFS or Li-Fraumeni-like syndrome is well known,4,6-10 these data establish a link between familial clustering of AML and TP53 germ line mutations for the first time. TP53 c.467G>C, p.R156P is a missense mutation that has been reported previously as a somatic event in different human malignancies. Importantly, it confers gain of function of p53 causing malignant transformation of hematopoietic cells in vitro. Together with the fact that loss of the wild-type allele was observed in leukemic specimens, these data suggest that it is indeed involved in AML development in this family.11 Data of murine leukemia models whereby AML development is significantly aggravated by aberrations in p53 further support this notion.12,13
An LFS pedigree showing for the first time familial clustering of AML. The index patient developed tAML (II:4) following cytotoxic treatment of diverse antecedent malignancies, and the index patient’s father (I:2) developed sAML following myelodysplasia. Filled symbols, subjects with malignancies; asterisk denotes a TP53 c.467G>C, p.R156P germ line mutation carrier; the “minus” denotes a wild-type TP53 germ line status. Numbers in brackets indicate age at diagnosis in years. BC, breast cancer; CML, chronic myeloid leukemia; CRC, colorectal cancer; PNET, primitive neuroectodermal tumor; TC, thyroid carcinoma.
An LFS pedigree showing for the first time familial clustering of AML. The index patient developed tAML (II:4) following cytotoxic treatment of diverse antecedent malignancies, and the index patient’s father (I:2) developed sAML following myelodysplasia. Filled symbols, subjects with malignancies; asterisk denotes a TP53 c.467G>C, p.R156P germ line mutation carrier; the “minus” denotes a wild-type TP53 germ line status. Numbers in brackets indicate age at diagnosis in years. BC, breast cancer; CML, chronic myeloid leukemia; CRC, colorectal cancer; PNET, primitive neuroectodermal tumor; TC, thyroid carcinoma.
To determine the frequency of TP53 germ line mutations in AML, a cohort of 186 patients with de novo AML (n = 72), secondary AML (sAML; n = 66), and tAML (n = 48) was investigated (supplemental Table 1). Exons 2-10 of TP53, including exon-intron boundaries, were analyzed by Sanger and targeted deep sequencing, respectively, using leukemic and constitutional DNA from either buccal swabs or remission material as reported earlier.14,15 Mutations were classified according to the “IACR TP53 Database” (http://p53.iarc.fr), “The TP53 Web Site” (http://p53.free.fr), and the “COSMIC database” (http://cancer.sanger.ac.uk/cosmic). Each TP53 mutation detected was confirmed. In case of a suspected germ line variant, DNA from a buccal swab obtained from patients in remission was used for this purpose. TP53 mutations were found in 35/186 (18.8%) diagnostic leukemia specimens with an equal distribution between different AML subtypes (de novo, 13/72 [18.1%]; sAML 14/66 [21.2%]; tAML 8/48 [16.7%]; P = .810 by Pearson’s χ2 test). The relatively high number of TP53 mutated AMLs in this cohort is due to an overrepresentation of high-risk cases and in concordance with published data.16 Most importantly, 2 further TP53 mutations of germ line origin were detected (2/186; 1.1%; c.673-1G>A and c.733G>A, respectively; supplemental Table 2), and again, both of them displayed a loss of the wild-type allele in leukemic specimens. Personal and family histories of the patient with the TP53 c.673-1G>A mutation fulfilled Chompret criteria3 as well (supplemental Figure 1). In this individual, multiplex testing of 150 genes associated with hereditary cancer has been performed previously revealing an additional CDH1 germ line variant.17 The fact of a cancer-associated germ line mutation not only is of relevance for genetic counseling of patients as well as family members but also has further consequences in the context of myeloid malignancies. AML patients with TP53 aberrations face an exceedingly poor prognosis and are candidates for allogeneic stem cell transplantation.18,19 Although no cases of transplantation of p53 mutant/deficient HSPCs have been reported yet, caution is, nevertheless, warranted based on experimental data. Transgenic mice with phosphorylation-site Trp53 mutations experience a depletion of adult stem cells, including those of the bone marrow. Furthermore, mice with germ line depletion of the Trp53 show—despite an expansion of bone marrow LSK cells—increased mortality rates when compared with Trp53-proficient animals.20,21
Interestingly, 2 of the 3 TP53 germ line–mutated patients described above suffered from tAML. As we already observed the occurrence of TP53 germ line mutations in 4/58 tAML patients previously,4 we extended our analyses within this subgroup by pooling these data with those of our index patient and the 48 tAML patients analyzed within the present study, thereby generating a cohort of 107 tAML patients with information available on the TP53 germ line mutation status as well as clinical parameters. In total, 6 out of 107 patients (5.6%) harbored a TP53 germ line mutation, which further highlights the importance of this genetic event in tAML (supplemental Tables 2 and 3). In light of the fact that HSPCs with TP53 aberrations have been shown to expand preferentially after cytotoxic treatment,22 these data suggest that TP53 germ line mutations could indeed predispose to the development of tAML. Interestingly, treatment of the primary malignancy included ionizing radiation in 5 out of 6 patients (83%) in contrast to 38/101 (38%) TP53 wild-type cases only (Table 1; P = .038 in Fisher’s exact test). In light of the fact that susceptibility to radiation-induced carcinogenesis has been shown in p53-deficient mouse models previously,23,24 one might speculate that human HSPCs carrying a TP53 germ line mutation might also respond inadequately to genotoxic stress induced by ionizing radiation. Despite this tempting assumption, it should be noted that the cohort of tAML patients studied is too small to draw a final conclusion, particularly as P values did not reach significance any longer when corrected for multiple hypothesis testing (Table 1). Therefore, analysis of larger tAML cohorts specifically addressing this issue will be needed to further corroborate such a correlation.
Clinical and laboratory characteristics of tAML patients according to the TP53 germ line status
| . | TP53 germ line status . | P value . | |
|---|---|---|---|
| Wild-type . | Mutated . | ||
| Number of patients (n) | 101 | 6 | .221 |
| Females/males, n (%) | 54 (53)/47 (47) | 5 (83)/1 (17) | |
| Age at diagnosis (y) | 65 (19-85) | 53 (8-80) | .263 |
| LDH (U/L) | 268 (121-2489) | 413 (127-543) | .948 |
| WBC at diagnosis (109/L) | 4.14 (0.81-305.7) | 2.62 (1.47-16.15) | .313 |
| Antecedent cytotoxic therapy, n (%) | |||
| Ionizing radiation | 38/101 (38) | 5/6 (83) | .038 |
| Alkylating agents | 48/101 (48) | 2/5 (40)* | .999 |
| Antimetabolites | 47/101 (47) | 1/5 (20)* | .374 |
| Topoisomerase inhibitors | 18/101 (18) | 2/5 (40)* | .237 |
| . | TP53 germ line status . | P value . | |
|---|---|---|---|
| Wild-type . | Mutated . | ||
| Number of patients (n) | 101 | 6 | .221 |
| Females/males, n (%) | 54 (53)/47 (47) | 5 (83)/1 (17) | |
| Age at diagnosis (y) | 65 (19-85) | 53 (8-80) | .263 |
| LDH (U/L) | 268 (121-2489) | 413 (127-543) | .948 |
| WBC at diagnosis (109/L) | 4.14 (0.81-305.7) | 2.62 (1.47-16.15) | .313 |
| Antecedent cytotoxic therapy, n (%) | |||
| Ionizing radiation | 38/101 (38) | 5/6 (83) | .038 |
| Alkylating agents | 48/101 (48) | 2/5 (40)* | .999 |
| Antimetabolites | 47/101 (47) | 1/5 (20)* | .374 |
| Topoisomerase inhibitors | 18/101 (18) | 2/5 (40)* | .237 |
One patient, whose chemotherapeutic agents administered for the primary malignancies were unknown, was excluded from statistical calculations.
LDH, lactate dehydrogenase; WBC, white blood cell count.
Cytotoxic therapies with leukemogenic potential were classified according to Sill et al.25
Taken together, these data demonstrate that TP53 germ line mutations occur in a small fraction of AML patients and are particularly frequent in tAML. Furthermore, they suggest a potential association of these aberrations with tAML occurring after ionizing radiation therapy, which will be of relevance for the design of future studies.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: This work was supported by Leukämiehilfe Steiermark (H.S.) and the Austrian Science Fund under grant no. P 26619-B19 (A.Z.).
Contribution: H.S. designed and conceived the study; A.Z., R.L., M.M., K.L., K.K., M.G., D.F., J.B.G., A.W., and H.S. acquired data; A.Z., R.L., M.M., K.L., K.K., M.G., D.F., J.B.G., A.W., and H.S. analyzed and interpreted data; A.Z. and H.S. wrote and reviewed the manuscript; all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heinz Sill, Medical University of Graz, Auenbruggerplatz 38, 8036 Graz, Austria; e-mail: heinz.sill@medunigraz.at.