Key Points
Fibrinogen γ-chain residues 390 to 396 bind FXIII-A2B2 and mediate its activation in a FXIII-B subunit-dependent mechanism.
Excess FXIII-B2 in plasma circulates bound to fibrinogen.
Abstract
Coagulation transglutaminase factor XIII (FXIII) exists in circulation as heterotetrameric proenzyme FXIII-A2B2. Effectively all FXIII-A2B2 circulates bound to fibrinogen, and excess FXIII-B2 circulates in plasma. The motifs that mediate interaction of FXIII-A2B2 with fibrinogen have been elusive. We recently detected reduced binding of FXIII-A2B2 to murine fibrinogen that has γ-chain residues 390-396 mutated to alanines (Fibγ390-396A). Here, we evaluated binding features using human components, including recombinant fibrinogen variants, FXIII-A2B2, and isolated FXIII-A2 and -B2 homodimers. FXIII-A2B2 coprecipitated with wild-type (γA/γA), alternatively-spliced (γ′/γ′), and αC-truncated (Aα251) fibrinogens, whereas coprecipitation with human Fibγ390-396A was reduced by 75% (P < .0001). Surface plasmon resonance showed γA/γA, γ′/γ′, and Aα251 fibrinogens bound FXIII-A2B2 with high affinity (nanomolar); however, Fibγ390-396A did not bind FXIII-A2B2. These data indicate fibrinogen residues γ390-396 comprise the major binding motif for FXIII-A2B2. Compared with γA/γA clots, FXIII-A2B2 activation peptide release was 2.7-fold slower in Fibγ390-396A clots (P < .02). Conversely, activation of recombinant FXIII-A2 (lacking FXIII-B2) was similar in γA/γA and Fibγ390-396A clots, suggesting fibrinogen residues γ390-396 accelerate FXIII-A2B2 activation in a FXIII-B2–dependent mechanism. Recombinant FXIII-B2 bound γA/γA, γ′/γ′, and Aα251 with similar affinities as FXIII-A2B2, but did not bind or coprecipitate with Fibγ390-396A. FXIII-B2 also coprecipitated with fibrinogen from FXIII-A–deficient mouse and human plasmas. Collectively, these data indicate that FXIII-A2B2 binds fibrinogen residues γ390-396 via the B subunits, and that excess plasma FXIII-B2 is not free, but rather circulates bound to fibrinogen. These findings provide insight into assembly of the fibrinogen/FXIII-A2B2 complex in both physiologic and therapeutic situations.
Introduction
Factor XIII (FXIII) is a plasma protransglutaminase that circulates at 14 to 28 µg/mL (43-86 nM; reviewed in Muszbek et al1 ). Zymogen FXIII is composed of 2 A subunits (FXIII-A2) and 2 carrier B subunits (FXIII-B2) assembled as a noncovalent heterotetramer (FXIII-A2B2). In plasma, FXIII-A2 is tightly associated (KD ∼100 pM)2 with FXIII-B2. Excess FXIII-B2 (43-62 nM) is present in circulation.2,3 During coagulation, FXIII-A2B2 is activated by thrombin-mediated cleavage of an N-terminal, 37-amino acid activation peptide from the FXIII-A subunits (FXIII-A2′). After activation peptide release, calcium promotes dissociation of the inhibitory FXIII-B subunits, yielding fully activated FXIII-A2* (FXIIIa). Once activated, FXIIIa catalyzes the formation of ε-N-(γ-glutamyl)-lysyl crosslinks between γ- and α-chains of fibrin and between fibrin and other plasma proteins. Crosslinking is essential for clot mechanical and biochemical stability (reviewed in Muszbek et al1 ). Fibrin α-chain crosslinking also promotes red blood cell retention in venous thrombi and, consequently, mediates thrombus composition and size.4,5
FXIII-A2B2 circulates in complex with fibrinogen (KD ∼10 nM),6 and these proteins are readily coprecipitated from plasma.7 However, the fibrinogen residues that mediate binding to FXIII-A2B2 in humans have not been defined. Early studies suggested the alternatively spliced fibrinogen γ′-chain contained the FXIII-A2B2–binding site.8,9 However, studies using recombinant fibrinogen showed that FXIII-A2B2 binds to γ- and γ′-containing fibrinogen with similar affinity,10 suggesting the γ′-extension is not necessary for FXIII-A2B2 binding. More recently, Smith et al observed high-affinity binding of FXIII-A2B2 to a glutathione-S-transferase–fused peptide containing amino acid residues 371-425 of the fibrinogen αC domain.11 However, whether the fibrinogen αC domain fulfills the carrier function of FXIII-A2B2 remains unclear.
We recently observed decreased coprecipitation of FXIII-A2B2 with murine fibrinogen that has alanine substitutions within residues γ390-396 (NRLSIGE to AAAAAAA, Fibγ390-396A), suggesting these γ-chain residues mediate the FXIII-A2B2 carrier function in mice.4 Accordingly, Souri et al subsequently detected binding of FXIII-A2B2 to the human fibrinogen γ-chain at residues C-terminal of γLys356.12 Notably, fibrinogen residues γ390-396 are highly conserved in mammals (NRLTIGE [human, gorilla, dog], NRLSIGD [rat], and NRLAIGE [giant panda]), suggesting these residues fulfill this function across species.
Herein, we used entirely human components, including recombinant human fibrinogen variants and human FXIII heterotetramers and homodimers, as well as FXIII-deficient mice, to define the interaction between these proteins. Our data reveal a direct interaction between human fibrinogen residues γ390-396 and the FXIII-B subunits, and uncover a fundamental mechanism mediating FXIII-fibrinogen complex assembly in blood. These data have important implications for both physiologic assembly of this complex in healthy individuals, and assembly in FXIII-A2–deficient patients receiving therapeutic recombinant FXIII-A2.
Methods
Proteins and materials
Anti-human fibrinogen antibody was from Dako (Carpinteria, CA) and Alexa Fluor 488 anti-rabbit and anti-sheep secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). Two rabbit polyclonal anti-FXIII-B antibodies were used: HPA003827 (Sigma-Aldrich, St. Louis, MO) and A074 (Zedira, Darmstadt, Germany), as indicated. Plasma FXIII-A2B2, anti-human FXIII-A antibody, and peak 1 human fibrinogen (FXIII-depleted) were from Enzyme Research Laboratories (South Bend, IN). Recombinant FXIII-A2 (rFXIII-A2) was a generous gift of Novo Nordisk (Bagsværd, Denmark). FXIII-A2B2 (plasma-derived) used for surface plasmon resonance (SPR) and recombinant FXIII-B2 (rFXIII-B2, produced in insect cells) were from Zedira. Insect cell-derived FXIII-B2 undergoes different posttranslational modification than human plasma-derived FXIII-B2 and migrates slightly faster on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Murine studies were approved by the Institutional Animal Care and Use Committees of the Cincinnati Children’s Hospital Medical Center and the University of North Carolina at Chapel Hill. Additional materials and sources are detailed in the supplemental Materials (available on the Blood Web site).
Expression of recombinant fibrinogen variants
A vector expressing recombinant Fibγ390-396A was constructed as detailed in supplemental Materials. Recombinant human wild-type (γA/γA), alternatively spliced (γ′/γ′), αC-truncated (Aα251), and Fibγ390-396A (Fibγ390-396A) fibrinogen variants were expressed in Chinese hamster ovary cells and affinity purified as previously described.13-15
Fibrinogen precipitation experiments
Fibrinogen (1 mg/mL [2.9 μM], final) was incubated with FXIII-A2B2 (20 μg/mL [60 nM], final), rFXIII-A2 (10 μg/mL [60 nM], final), or rFXIII-B2 (10 μg/mL [63 nM], final) at room temperature for 15 minutes. Glycine (165 mg/mL) was then added and samples were rotated for 1 hour, after which the precipitate was pelleted by centrifugation (7000g, 15 minutes) and resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered saline (HBS; 20 mM HEPES [pH 7.4], 150 mM NaCl). Fibrinogen, FXIII-A, and FXIII-B content of the initial sample, pellet, and supernatant were assessed by western blotting under reducing conditions, unless otherwise specified. Briefly, samples were separated by SDS-PAGE on 10% Tris-glycine gels, transferred to polyvinylidene fluoride membranes, and probed with primary antibodies (rabbit anti-human fibrinogen [1:7000], sheep anti-human FXIII-A [1:1000], or rabbit anti-human FXIII-B [HPA003827, 1:500]) overnight at 4°C before incubation with fluorescently labeled secondary antibodies for 1 hour at room temperature. Blots were visualized using a Typhoon FLA9000 Imager (GE Healthcare, Little Chalfont, United Kingdom). Densitometry was performed with ImageJ 1.48v. The percentage of FXIII-A or FXIII-B in the pellet was determined by dividing the intensity of the subunit band in the pellet by the sum of the band intensities in both the pellet and the supernatant.
For experiments with mouse plasma, blood was drawn via the inferior vena cava and processed to platelet-poor plasma by centrifugation (5000g, 10 minutes). Fibrinogen was isolated from wild-type (F13a+/+), heterozygous (F13a+/−), FXIII-deficient (F13a−/−),4,16 or afibrinogenemic17 mouse plasma by glycine precipitation.4 For experiments with immunodepleted human plasma (Affinity Biologicals, Ancaster, ON, Canada; lacks both FXIII-A and FXIII-B), plasma was first reconstituted with 10 μg/mL rFXIII-B2 and diluted threefold prior to glycine precipitation. Precipitated fibrinogen and FXIII-A were detected as in the previous paragraph. Both murine and human FXIII-B were detected using anti-human FXIII-B antibody (A074, 1:1000 overnight at room temperature).
Surface plasmon resonance
SPR ligands and analytes were prepared as detailed in supplemental Materials. SPR was performed on a SensiQ Pioneer platform described in supplemental Materials. Fibrinogen analytes were diluted to 50 nM or 1 µM using the same batch of running buffer for blanks and the OneStep titration function. Fibrinogen analytes were injected into the sensor chamber at a flow rate of 30 µL per minute using the OneStep titration function18,19 with a loop inject of 75% following 5 leadoff blanks and 3 bulk standard injections of 3% sucrose in running buffer. The 50-nM and 1-µM samples had dissociation times of 500 and 1000 seconds, respectively. The chip surface was regenerated with 2 M NaCl (30 µL per minute, 60 seconds), followed by 3 M NaCl (30 µL per minute, 60 seconds).
Data were analyzed with Qdat data analysis software (SensiQ Technologies Inc, Oklahoma City, OK). Binding data were fit using a simple ka/kd model and aggregation/retention parameters adjusted per binding curve according to goodness of fit and curve type. Sensorgrams from experiments with 50 nM fibrinogen analytes (maximum) recognized 1 binding site and were fit using a 1-site model. Sensorgrams with 1 µM fibrinogen analyte (maximum) identified 2 binding sites for some FXIII ligands, so these data were fit using a 2-site binding model; however, given the plasma concentrations of FXIII-A2B2 and fibrinogen, affinities for the second binding sites (0.7-21 µM) were considered too weak to be physiologically relevant and are not reported.
FXIII activation and fibrin crosslinking
FXIII-A2B2 (20 μg/mL [60 nM], final) or rFXIII-A2 (10 μg/mL [60 nM], final) were incubated with fibrinogen (0.15 mg/mL [440 nM], final) at room temperature for 15 minutes. Reactions were triggered with thrombin (2 nM, final) and calcium (10 mM, final). This low thrombin concentration enabled us to detect early FXIII activation and fibrin crosslinking. Reactions were quenched and clots dissolved with 50 mM dithiothreitol/12.5 mM EDTA in 8 M urea (60°C, 1 hour). Samples were boiled in SDS-containing sample buffer and separated using SDS-PAGE on 10% Tris-glycine gels before transfer to polyvinylidene fluoride membranes. Membranes were probed with primary antibodies (sheep anti-human FXIII-A [1:1000] or rabbit anti-human fibrinogen [1:7000]; overnight, 4°C) before incubation with fluorescently labeled secondary antibodies (1 hour, room temperature). Blots were visualized using a Typhoon FLA9000 Imager. Densitometry was performed with ImageJ 1.48v. FXIII activation was determined by dividing the intensity of the FXIII-A′ band by the sum of the FXIII-A and FXIII-A′ bands to obtain the percentage of FXIII-A′. Crosslinking of fibrin γ-chains was determined as previously described.12,20
Statistics
Descriptive statistics (mean, standard deviation, standard error of the mean [SEM]) were calculated and the Lilliefors test was used to assess normality. FXIII-A2 and FXIII-B2 coprecipitation with each fibrinogen variant was compared with γA/γA using analysis of variance with Dunnett post hoc testing (Kaleidagraph, Synergy Software, v4.5). FXIII activation and fibrin crosslinking rates were compared using 2-tailed Student t tests for equal or unequal variances, as appropriate. P < .05 was considered statistically significant.
Results
Fibrinogen residues γ390-396 mediate FXIII-A2B2 binding to soluble human fibrinogen
Previous studies have suggested FXIII-A2B2 binds to fibrinogen at the alternatively spliced γ′-chain,8,9 residues in the αC domain,11 or residues in the γ-chain.4,12 To compare FXIII-A2B2 binding to these regions of fibrinogen, we expressed and purified recombinant human fibrinogen proteins: wild-type (γA/γA), alternatively spliced (γ′/γ′), αC-truncated (Aα251), and Fibγ390-396A. As expected,13-15,21 each of these fibrinogen variants contained all 3 chains, polymerized normally, and were >95% clottable (Figure 1 and data not shown). We preincubated these fibrinogen variants with FXIII-A2B2, precipitated fibrinogen with glycine, and performed SDS-PAGE and western blotting to identify FXIII-A2B2 present in the pellets and supernatant. FXIII-A2B2 coprecipitated with γA/γA, γ′/γ′, and Aα251 fibrinogen constructs (Figure 1). However, coprecipitation of FXIII-A2B2 with Fibγ390-396A was reduced by 75% (P < .0001; Figure 1).
Fibrinogen residues γ390-396 are necessary for FXIII-A2B2 binding. Recombinant human fibrinogen variants (γA/γA, γ′/γ′, Aα251, and Fibγ390-396A) were mixed with FXIII-A2B2 (1 mg/mL [2.9 μM] and 20 µg/mL [60 nM], final, respectively) and precipitated with glycine. (A) Representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial sample (I), pellet (P), or supernatant (S). Note that P and S samples were prepared after the addition of glycine and are therefore diluted relative to the I sample. (B) Quantitation of all blots, indicating percent of FXIII-A or FXIII-B in the pellet, relative to total FXIII in the pellet and supernatant. Bars are means ± SEM; N = 3.
Fibrinogen residues γ390-396 are necessary for FXIII-A2B2 binding. Recombinant human fibrinogen variants (γA/γA, γ′/γ′, Aα251, and Fibγ390-396A) were mixed with FXIII-A2B2 (1 mg/mL [2.9 μM] and 20 µg/mL [60 nM], final, respectively) and precipitated with glycine. (A) Representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial sample (I), pellet (P), or supernatant (S). Note that P and S samples were prepared after the addition of glycine and are therefore diluted relative to the I sample. (B) Quantitation of all blots, indicating percent of FXIII-A or FXIII-B in the pellet, relative to total FXIII in the pellet and supernatant. Bars are means ± SEM; N = 3.
We also quantified binding of the fibrinogen variants to FXIII-A2B2 using SPR. Consistent with the coprecipitation data, γA/γA and γ′/γ′ fibrinogens bound FXIII-A2B2 with similar, high affinity (Table 1; supplemental Figure 1). Compared with γA/γA and γ′/γ′ fibrinogens, Aα251 fibrinogen binding to adherent FXIII-A2B2 was slightly reduced, consistent with the finding that the αC domain contributes to FXIII-A2B2–binding function.11 Regardless, given these affinity constants (Table 1), >99% of circulating FXIII-A2B2 (plasma concentration ∼43-86 nM2,3 ) would bind each of these variants. In contrast, Fibγ390-396A fibrinogen did not bind FXIII-A2B2 (Table 1; supplemental Figure 1). Collectively, these data indicate the primary binding site for zymogen FXIII-A2B2 is present in γA/γA, γ′/γ′, and Aα251 fibrinogens, but absent in Fibγ390-396A fibrinogen. Together with previous findings,4 these results suggest the highly conserved fibrinogen residues γ390-396 mediate binding of zymogen FXIII-A2B2 to fibrinogen in both mice and humans.
SPR analysis of fibrinogen variant binding to immobilized FXIII-A2B2 and rFXIII-B2
| Immobilized ligand . | Analyte . | N . | Equilibrium dissociation constant (KD), nM . |
|---|---|---|---|
| FXIII-A2B2 | γA/γA | 6 | 3.8 ± 2.4 |
| γʹ/γʹ | 6 | 10.4 ± 11.1 | |
| Aα251 | 3 | 71.0 ± 16.2 | |
| Fibγ390-396A | 6 | No binding | |
| rFXIII-B2 | γA/γA | 4 | 0.4 ± 0.3 |
| γʹ/γʹ | 6 | 53.0 ± 75.1 | |
| Aα251 | 4 | 58.6 ± 26.5 | |
| Fibγ390-396A | 6 | No binding |
| Immobilized ligand . | Analyte . | N . | Equilibrium dissociation constant (KD), nM . |
|---|---|---|---|
| FXIII-A2B2 | γA/γA | 6 | 3.8 ± 2.4 |
| γʹ/γʹ | 6 | 10.4 ± 11.1 | |
| Aα251 | 3 | 71.0 ± 16.2 | |
| Fibγ390-396A | 6 | No binding | |
| rFXIII-B2 | γA/γA | 4 | 0.4 ± 0.3 |
| γʹ/γʹ | 6 | 53.0 ± 75.1 | |
| Aα251 | 4 | 58.6 ± 26.5 | |
| Fibγ390-396A | 6 | No binding |
Equilibrium dissociation constant values are mean ± standard deviation for the number of experiments indicated (N).
Human fibrin(ogen) residues γ390-396 mediate the ability of fibrin to accelerate FXIII-A2B2 activation
During fibrin formation, FXIII-A2B2 binding to the fibrin D:E:D/thrombin complex accelerates FXIII activation peptide cleavage and FXIII activation.12,22-28 Using mice expressing murine Fibγ390-396A fibrinogen, we previously detected delayed FXIII activation and fibrin crosslinking in plasma.4 To directly determine the contribution of fibrin(ogen) residues γ390-396 to these functions, we now assessed the ability of human Fibγ390-396A fibrin(ogen) to support FXIII-A2B2 activation in a purified system. Compared with reactions with human γA/γA fibrin(ogen), reactions with Fibγ390-396A fibrin(ogen) showed 2.7-fold slower release of the FXIII activation peptide (2.35% ± 0.46% vs 0.87% ± 0.20% FXIII-A′ per minute, respectively, P < .02; Figure 2A-C), and was similar to that observed in the absence of fibrinogen (0.96% ± 0.16% FXIII-A′ per minute, P = .8; Figure 2A-C).
Fibrin(ogen) residues γ390-396 mediate the acceleratory effect of fibrin(ogen) on FXIII-A2B2 activation. FXIII-A2B2 (20 µg/mL [60 nM], final) was mixed with recombinant fibrinogens (γA/γA or Fibγ390-396A, 150 µg/mL [440 nM], final) or buffer (No Fgn). Reactions were triggered by addition of thrombin (2 nM, final) and CaCl2 (10 mM, final), quenched at the indicated time points, and analyzed by SDS-PAGE with western blotting and densitometry. Activation peptide cleavage was detected using anti-FXIII-A antibody. Fibrin crosslinking was detected using anti-fibrinogen antibody. (A) Representative western blots and (B) quantitation of FXIII-A2B2 activation over time from all blots. (C) Maximal rates of FXIII-A2B2 activation calculated from panel B. (D) Representative western blots of fibrin crosslinking, and quantitation of (E) γ-γ dimer formation and (F) γ-γ dimer formation rate. Data are means ± SEM; N = 3-6 replicates per time point.
Fibrin(ogen) residues γ390-396 mediate the acceleratory effect of fibrin(ogen) on FXIII-A2B2 activation. FXIII-A2B2 (20 µg/mL [60 nM], final) was mixed with recombinant fibrinogens (γA/γA or Fibγ390-396A, 150 µg/mL [440 nM], final) or buffer (No Fgn). Reactions were triggered by addition of thrombin (2 nM, final) and CaCl2 (10 mM, final), quenched at the indicated time points, and analyzed by SDS-PAGE with western blotting and densitometry. Activation peptide cleavage was detected using anti-FXIII-A antibody. Fibrin crosslinking was detected using anti-fibrinogen antibody. (A) Representative western blots and (B) quantitation of FXIII-A2B2 activation over time from all blots. (C) Maximal rates of FXIII-A2B2 activation calculated from panel B. (D) Representative western blots of fibrin crosslinking, and quantitation of (E) γ-γ dimer formation and (F) γ-γ dimer formation rate. Data are means ± SEM; N = 3-6 replicates per time point.
The rate of fibrin formation was similar for γA/γA and Fibγ390-396A (Figure 2D and data not shown). However, the rate of fibrin crosslinking was slower for Fibγ390-396A. Specifically, γ-γ dimer formation was 2.5-fold slower (6.7% ± 1.5% vs 17.0% ± 1.3% γ-γ per minute, for Fibγ390-396A vs γA/γA, respectively, Figure 2D-F). The delays in both FXIII-A2B2 activation and crosslinking activity are consistent with a lack of binding of zymogen FXIII-A2B2 to Fibγ390-396A fibrinogen.
The ability of fibrin(ogen) residues γ390-396 to accelerate FXIII activation is FXIII-B subunit-dependent
Souri et al previously suggested the acceleratory effect of fibrin(ogen) on FXIII-A2B2 activation is FXIII-B subunit-dependent.12 We therefore also measured activation of FXIII-A2 (rFXIII-A2) in the absence of FXIII-B2 and compared these rates in the presence of γA/γA and Fibγ390-396A fibrin(ogen). Compared with activation of FXIII-A2B2, activation of rFXIII-A2 was slower (Figures 2A-C and 3A-C; P < .02), consistent with a critical role for the FXIII-B subunits in this reaction. Interestingly, however, in contrast to that seen with FXIII-A2B2, activation of rFXIII-A2 was similar in the presence of γA/γA and Fibγ390-396A fibrin(ogen) (0.60% ± 0.06% vs 0.46% ± 0.06% FXIII-A′ per minute, respectively, Figure 3A-C). Moreover, in the presence of rFXIII-A2, the formation rate of γ-γ dimers was more similar for γA/γA and Fibγ390-396A (15.6% ± 0.5% vs 12.6% ± 0.5% γ-γ per minute, respectively, Figure 3D-F), relative to reactions in the presence of FXIII-A2B2. These findings show Fibγ390-396A can be crosslinked, indicating the delayed crosslinking seen with FXIII-A2B2 (Figure 2D-F) was not due to a substantial disruption of structure in this region. Rather, these data attribute the delay seen with FXIII-A2B2 (Figure 2D-F) to decreased interaction between fibrin(ogen) residues γ390-396 and the FXIII-B subunit. Together, these data suggest fibrin(ogen) residues γ390-396 accelerate FXIII activation, and do so in a FXIII-B subunit-dependent mechanism.
Fibrin(ogen) residues γ390-396 do not accelerate FXIII-A2 activation. rFXIII-A2 (10 µg/mL [60 nM], final) was mixed with γA/γA or Fibγ390-396A fibrinogen (150 µg/mL [440 nM], final). Reactions were triggered by addition of thrombin (2 nM, final) and CaCl2 (10 mM, final), quenched at the indicated time points, and analyzed by SDS-PAGE with western blotting and densitometry. Activation peptide cleavage was detected using anti-FXIII-A antibody. Fibrin crosslinking was detected using anti-fibrinogen antibody. (A) Representative western blots and (B) quantitation of rFXIII-A2 activation over time from all blots. (C) Maximal rates of rFXIII-A2 activation were calculated from quantified western blots. (D) Representative western blots of fibrin crosslinking and quantification of (E) γ-γ dimer formation and (F) formation rate. Data are means ± SEM; N = 4 experiments.
Fibrin(ogen) residues γ390-396 do not accelerate FXIII-A2 activation. rFXIII-A2 (10 µg/mL [60 nM], final) was mixed with γA/γA or Fibγ390-396A fibrinogen (150 µg/mL [440 nM], final). Reactions were triggered by addition of thrombin (2 nM, final) and CaCl2 (10 mM, final), quenched at the indicated time points, and analyzed by SDS-PAGE with western blotting and densitometry. Activation peptide cleavage was detected using anti-FXIII-A antibody. Fibrin crosslinking was detected using anti-fibrinogen antibody. (A) Representative western blots and (B) quantitation of rFXIII-A2 activation over time from all blots. (C) Maximal rates of rFXIII-A2 activation were calculated from quantified western blots. (D) Representative western blots of fibrin crosslinking and quantification of (E) γ-γ dimer formation and (F) formation rate. Data are means ± SEM; N = 4 experiments.
The FXIII-B subunit binds fibrinogen residues γ390-396
We then directly tested the hypothesis that FXIII binding to fibrinogen residues γ390-396 is mediated by the B subunits using both precipitation and SPR assays. First, we precipitated recombinant γA/γA or Fibγ390-396A fibrinogen in the presence of rFXIII-A2, rFXIII-B2, or rFXIII-A2 plus rFXIII-B2. rFXIII-A2 did not coprecipitate with either γA/γA or Fibγ390-396A fibrinogen (Figure 4). However, rFXIII-B2 readily coprecipitated with γA/γA fibrinogen (Figure 4A), but not Fibγ390-396A fibrinogen (Figure 4B). Addition of rFXIII-B2 to rFXIII-A2 rescued coprecipitation of rFXIII-A2 with γA/γA fibrinogen (Figure 4A), likely through the formation of rFXIII-A2B2 heterotetramers. Conversely, rFXIII-B2 did not rescue rFXIII-A2 coprecipitation with Fibγ390-396A (Figure 4B). Experiments using purified, plasma-derived γA/γA (peak 1) fibrinogen fully recapitulated findings with recombinant γA/γA (data not shown).
FXIII-A2B2 binds fibrinogen residues γ390-396 via the FXIII-B subunits. rFXIII-A2 (10 µg/mL [60 nM], final), rFXIII-B2 (10 µg/mL [62 nM], final), or both, were mixed with (A) γA/γA or (B) Fibγ390-396A fibrinogen (1 mg/mL [2.9 μM], final) and precipitated with glycine. (A-B) Representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial sample (I), pellet (P), or supernatant (S). Note that P and S samples were prepared after the addition of glycine and are therefore diluted relative to the I sample. Blots are representative of N = 3 experiments.
FXIII-A2B2 binds fibrinogen residues γ390-396 via the FXIII-B subunits. rFXIII-A2 (10 µg/mL [60 nM], final), rFXIII-B2 (10 µg/mL [62 nM], final), or both, were mixed with (A) γA/γA or (B) Fibγ390-396A fibrinogen (1 mg/mL [2.9 μM], final) and precipitated with glycine. (A-B) Representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial sample (I), pellet (P), or supernatant (S). Note that P and S samples were prepared after the addition of glycine and are therefore diluted relative to the I sample. Blots are representative of N = 3 experiments.
Second, we examined binding of the fibrinogen variants to surface-bound rFXIII-B2. These data revealed that γA/γA bound rFXIII-B2 with similar affinity as seen with FXIII-A2B2 (Table 1, supplemental Figure 2). γ′/γ′ and Aα251 fibrinogens also bound rFXIII-B2 (Table 1, supplemental Figure 2), indicating both that the binding motif for FXIII-B2 is present on each of these fibrinogen molecules and that the presence of FXIII-A2 does not enhance FXIII-B2 binding to fibrinogen. These data also confirm that rFXIII-B2 coprecipitation with fibrinogen in the previous experiments was not due to the presence of glycine. Notably, however, we were unable to detect binding of Fibγ390-396A fibrinogen to rFXIII-B2 (Table 1; supplemental Figure 2). Collectively, these coprecipitation and SPR data indicate that the FXIII-B subunit(s) of FXIII-A2B2 mediate binding to fibrinogen residues γ390-396.
Excess FXIII-B2 in plasma circulates bound to fibrinogen
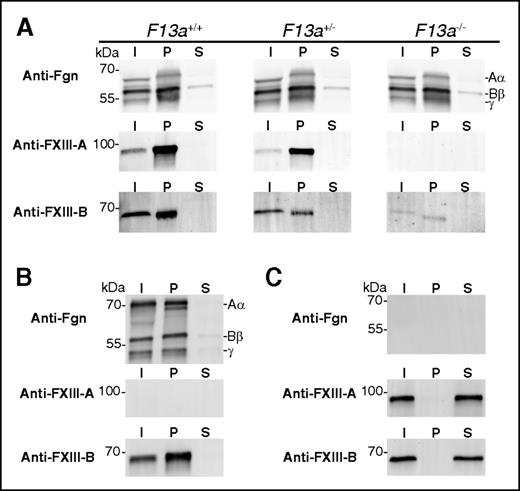
FXIII-B2 is present in approximately twofold molar excess over FXIII-A2 in plasma and is reported to circulate as free (unbound) FXIII-B2 homodimer.2,3 However, the coprecipitation and SPR data indicating FXIII-B2 can bind fibrinogen in the absence of FXIII-A2 (Figure 4A; Table 1) raise the interesting possibility that “free” FXIII-B2 in plasma actually circulates bound to fibrinogen. Indeed, given the measured affinity of FXIII-B2 to fibrinogen (0.4 nM, Table 1) and the estimated plasma concentration of “free” FXIII-B2 (∼43-62 nM),2,3 >99% of circulating FXIII-B2 should be bound to fibrinogen. Therefore, to determine whether FXIII-B2 circulates with fibrinogen in plasma, we precipitated fibrinogen from plasma from FXIII-A2–sufficient (F13a+/+) and FXIII-A2–deficient (F13a+/− and F13a−/−) mice4,16 and used SDS-PAGE and western blotting to detect FXIII-B in the precipitate. Consistent with previous observations,29 there was a FXIII-A subunit dose effect on the total amount of FXIII-B present in the initial plasma sample (Figure 5A), suggesting FXIII-A2 influences the circulating level of FXIII-B2. Regardless, Figure 5A shows that FXIII-B2 coprecipitated with fibrinogen from FXIII-A–deficient plasma. Experiments using FXIII-depleted human plasma reconstituted with rFXIII-B2 fully recapitulated findings with FXIII-A–deficient mouse plasma (Figure 5B). To test the specificity of the precipitation protocol, we subjected afibrinogenemic mouse plasma to glycine precipitation. As expected, no fibrinogen was precipitated (Figure 5C). Importantly, glycine did not precipitate FXIII-B2 from afibrinogenemic mouse plasma (Figure 5C), indicating that FXIII precipitation in these experiments is fibrinogen-dependent. Thus, these data suggest excess FXIII-B2 in plasma does not circulate in a “free” state, but instead circulates bound to fibrinogen.
In the absence of FXIII-A2, FXIII-B2 coprecipitates with plasma fibrinogen. Fibrinogen was precipitated from (A) F13a+/+, F13a+/−, or F13a−/− mouse plasma, (B) FXIII-depleted human plasma reconstituted with rFXIII-B2, or (C) afibrinogenemic mouse plasma using glycine. Panels show representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial plasma (I), pellet (P), or supernatant (S) under reducing (Fgn) or nonreducing (FXIII-A, FXIII-B) conditions. Note in panel A, plasma albumin in I and S samples causes the Aα-chain to migrate faster than in the P samples. Blots are representative of N = 3 experiments with mouse plasmas and N = 2 experiments with human plasma.
In the absence of FXIII-A2, FXIII-B2 coprecipitates with plasma fibrinogen. Fibrinogen was precipitated from (A) F13a+/+, F13a+/−, or F13a−/− mouse plasma, (B) FXIII-depleted human plasma reconstituted with rFXIII-B2, or (C) afibrinogenemic mouse plasma using glycine. Panels show representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial plasma (I), pellet (P), or supernatant (S) under reducing (Fgn) or nonreducing (FXIII-A, FXIII-B) conditions. Note in panel A, plasma albumin in I and S samples causes the Aα-chain to migrate faster than in the P samples. Blots are representative of N = 3 experiments with mouse plasmas and N = 2 experiments with human plasma.
Discussion
The observation that FXIII-A2B2 circulates in complex with fibrinogen is well established; however, the motifs on fibrinogen and FXIII-A2B2 that mediate this interaction have been controversial. Our study that integrates both solid- and solution-phase binding experiments and functional assays reveals critical components of both fibrinogen and FXIII-A2B2 necessary for binding. First, our data indicate that the primary binding site for zymogen FXIII-A2B2 on human fibrinogen is not found in the alternatively spliced γ′-extension or the αC region, but instead lies within γ-chain residues 390-396. Together with data from mice,4 this finding suggests these highly conserved fibrinogen residues mediate this interaction in multiple species. Second, we showed that FXIII-A2B2 binds fibrinogen residues γ390-396 via the FXIII-B subunits. These data support previous studies suggesting an interaction between the FXIII-B subunit and fibrinogen,8,12 and extend these findings by defining the FXIII-B binding motif on fibrinogen. Third, we showed that FXIII-B2 can bind fibrinogen in the absence of FXIII-A2. This intriguing finding suggests “free” FXIII-B2 in plasma actually circulates bound to fibrinogen, and has important implications for understanding assembly of the fibrinogen/FXIII-A2B2 complex in both physiologic and therapeutic situations.
Our finding that fibrinogen residues γ390-396 support FXIII-A2B2 binding are consistent with several prior studies implicating the D domain,30 residues C-terminal to residue Lys356 of the γ-chain,12 and residues γ390-396 of murine fibrinogen4 in this interaction. However, our findings are discordant with studies implicating the alternatively spliced γ′-chain8,9 as the primary mediator of this interaction. The reasons for this discord may relate to differences in the assay systems used. For example, although experiments using anion exchange chromatography suggested FXIII-A2B2 elutes in the same fraction as γA/γ′, this study did not directly compare binding of FXIII-A2B2 to these fibrinogen variants.8 In contrast, we tested this interaction by both coprecipitation and direct binding assays that enabled us to maintain fibrinogen in solution during the binding events. This assay design may be particularly important because the same residues we have implicated in FXIII-A2B2 binding have previously been shown to support fibrin(ogen) binding to the CD11b (αM) subunit of CD11b/CD18 (Mac-1) integrin present on monocytes, macrophages, and neutrophils.21,31 The observation from those studies that CD11b binds to residues γ390-396 in insoluble fibrin and adherent fibrin(ogen), but not soluble fibrinogen, has led to the hypothesis that fibrin formation or fibrinogen adherence to a surface induces structural changes within these residues. Thus, previous experimental designs that used surface (or resin)-bound fibrin(ogen) or fibrin6,8,26,32,33 may not have recapitulated the conformation of residues γ390-396 that would bind zymogen FXIII when fibrinogen is in solution. This possibility is also interesting when considering the role of the nearby fibrin(ogen) γ′ extension (residues 407-427) in FXIII-A2B2 binding. If residues in the γ′ extension influence structure within residues γ390-396, data from assays using surface- or resin-bound fibrin(ogen) or fibrin may have indicated the alternatively spliced γ′ chain has different affinity for FXIII-A2B2. Similarly, ultracentrifugation experiments with γA/γ′ fibrinogen9 may have been confounded by the presence of gel-like fibrin(ogen) dimers.34,35 Thus, these conditions may have led to the conclusion that the zymogen-binding motif is contained within the γ′ extension.
More recent findings that FXIII-A2B2 binds to a peptide derived from the fibrinogen αC domain (residues α371-425) suggested FXIII-A2B2 binds to the fibrinogen αC region.11 In that study,11 it was not possible to distinguish the relative contributions of the αC region vs the D region to this interaction in full-length fibrinogen. Our SPR data indicating Aα251 fibrinogen has weaker binding than γA/γA fibrinogen to FXIII-A2B2 suggests some FXIII-binding character is derived from the αC domain. Future studies using variant fibrinogens with combined mutations in the γ- and α-chains may resolve the relative contribution of the αC region in this interaction.
The high degree of interspecies homology within fibrinogen residues γ390-396 has traditionally been attributed to their other essential function in supporting fibrin(ogen) binding to CD11b.21,31 Interestingly, the earliest FXIII-fibrinogen system36 and the αM I domain that binds fibrin37,38 appeared together with the rise of vertebrates over 400 million years ago. No homozygous mutations have been identified in this region, emphasizing the physiologic importance of this fibrin(ogen) sequence. Therefore, the high homology in this region may result from strong evolutionary pressure to maintain both of these functions. Although the same fibrinogen residues mediate binding to both FXIII-A2B2 and CD11b, it is unlikely that these binding events compete. First, there is a vast excess of fibrinogen relative to FXIII-A2B2. Second, FXIII-A2B2 circulates with soluble fibrinogen,6 whereas CD11b binds insoluble fibrin.39 Thus, these interactions likely occur in distinct physiologic settings.
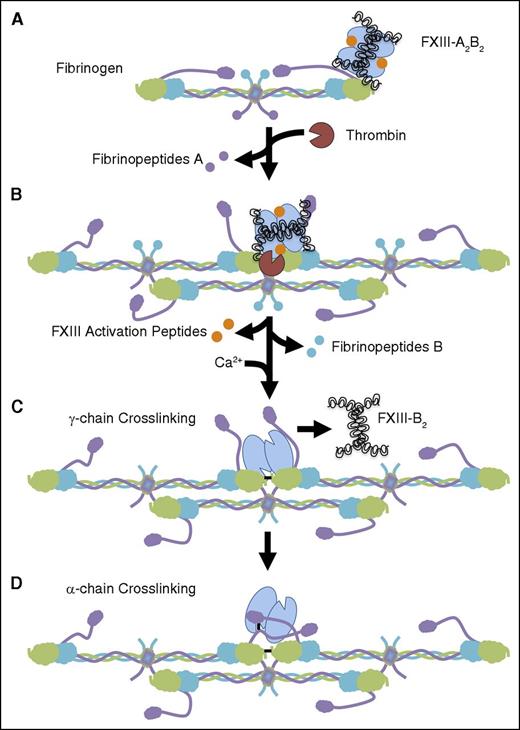
Based on our and published findings, we propose the following model (Figure 6). FXIII-A2B2 circulates bound to fibrinogen at a site composed of residues γ390-396 and supported by the αC region.11 During coagulation, fibrinogen transports FXIII-A2B2 into the nascent clot via its interaction with the FXIII-B subunits. As fibrin polymerizes, FXIII-A2B2 bound to the D domain of 1 fibrin monomer contacts thrombin bound to the E domain of another fibrin monomer at the D:E:D interface.40 Formation of this complex promotes FXIII activation peptide cleavage and release from the FXIII-A subunits,22-28,40 followed by dissociation of the FXIII-B subunits from the FXIII-A subunits.12,22 These sequential steps yield fully activated FXIII-A2*. Generation of FXIII-A2* at residues γ390-396 conveniently localizes FXIIIa near the γ-chain crosslinking sites (residues γQ398/399 and γK406), which are the first fibrin residues to undergo crosslinking.20,41-43 This γ-chain crosslinking also promotes FXIII-B2 dissociation from the fibrin clot.12 The FXIII-A2* interaction with αC residue E39611,44,45 then facilitates the translocation of its active site to the fibrin α-chain, where it catalyzes the formation of crosslinks between fibrin α-chains and between α-chains and other plasma proteins. Formation of these crosslinks is critical for the ability of FXIII(a) to promote resistance of clots to biomechanical and biochemical disruption. Moreover, we recently showed that the spatiotemporal regulation of FXIII activation kinetics during coagulation is also critical for determining red blood cell retention in contracted clots.4 Thus, this model reconciles data from studies on the binding, activation, and activity of FXIII and reveals the importance of the FXIII-B subunits in fibrinogen/FXIII-A2B2 interactions, in FXIII-A2B2 activation, and consequently, in fibrin crosslinking and clot composition and stability. Although we did not identify the specific FXIII-B residues that mediate this interaction, previous studies have implicated sushi domains 1 and/or 10 in this interaction.12 Further studies are ongoing to localize the FXIII-B residues that support binding to fibrinogen residues γ390-396.
FXIII-A2B2 binding to fibrinogen residues γ390-396 promotes FXIII-A2 B2 activation and activity. (A) Fibrinogen is composed of 2 Aα- (purple), 2 Bβ- (teal), and 2 γ-chains (green) arranged in a trinodular structure with 2 distal D domains and a central E domain. The Aα-chains have a C-terminal domain (αC) that extends beyond the D domain. FXIII-A2B2 circulates bound to fibrinogen γ-chain residues 390-396 via the FXIII-B subunits. (B) Once coagulation is initiated, thrombin interacts with the fibrinogen E domain and cleaves fibrinopeptides A from the Aα-chains. As fibrin monomers polymerize, FXIII-A2B2 associated with γ390-396 is brought into contact with thrombin at the D:E:D interface to form a ternary complex.40 This complex facilitates thrombin-mediated activation peptide cleavage from the FXIII-A subunits.22-28,40 (C) Following activation peptide cleavage, fibrin promotes the calcium-mediated FXIII-B subunit dissociation from the FXIII-A subunits to yield FXIIIa.12,22 FXIIIa then crosslinks (black line) the nearby γ-chains yielding γ-γ dimers.20 This γ-chain crosslinking also promotes dissociation of FXIII-B2 from the fibrin clot.12 (D) FXIIIa translocates from the γ-chain to the αC region, binding at or near α-chain residue E396,11,44,45 and catalyzes the formation of crosslinks between fibrin α-chains and between fibrin and other plasma proteins.
FXIII-A2B2 binding to fibrinogen residues γ390-396 promotes FXIII-A2 B2 activation and activity. (A) Fibrinogen is composed of 2 Aα- (purple), 2 Bβ- (teal), and 2 γ-chains (green) arranged in a trinodular structure with 2 distal D domains and a central E domain. The Aα-chains have a C-terminal domain (αC) that extends beyond the D domain. FXIII-A2B2 circulates bound to fibrinogen γ-chain residues 390-396 via the FXIII-B subunits. (B) Once coagulation is initiated, thrombin interacts with the fibrinogen E domain and cleaves fibrinopeptides A from the Aα-chains. As fibrin monomers polymerize, FXIII-A2B2 associated with γ390-396 is brought into contact with thrombin at the D:E:D interface to form a ternary complex.40 This complex facilitates thrombin-mediated activation peptide cleavage from the FXIII-A subunits.22-28,40 (C) Following activation peptide cleavage, fibrin promotes the calcium-mediated FXIII-B subunit dissociation from the FXIII-A subunits to yield FXIIIa.12,22 FXIIIa then crosslinks (black line) the nearby γ-chains yielding γ-γ dimers.20 This γ-chain crosslinking also promotes dissociation of FXIII-B2 from the fibrin clot.12 (D) FXIIIa translocates from the γ-chain to the αC region, binding at or near α-chain residue E396,11,44,45 and catalyzes the formation of crosslinks between fibrin α-chains and between fibrin and other plasma proteins.
A major finding from this work is the observation that FXIII-B2 can bind fibrinogen in the absence of FXIII-A2. Notably, the tight affinity of FXIII-B2 binding to fibrinogen, together with the plasma concentrations of FXIII-B2 and fibrinogen, suggests that essentially all FXIII-B2 in plasma is bound to fibrinogen. This observation appears to contradict the tenet that “free B” circulates in plasma.3 However, FXIII-B2 used in the previous report3 was prepared by ammonium sulfate precipitation and heat denaturation to specifically remove fibrinogen. Thus, that study was not designed to characterize FXIII binding to other plasma proteins, and “free B” was likely only meant to imply “not bound to FXIII-A2.” This nomenclature has been interpreted overly broadly since that time. Because both FXIII-B2 and fibrinogen are synthesized by hepatocytes, these proteins may associate during or immediately following their secretion. Subsequent association of the FXIII-A2 subunits, which are synthesized by cells of bone marrow origin, with the fibrinogen/FXIII-B2 complex would then result in formation of the complete fibrinogen/FXIII-A2B2 complex. Consequently, our data that suggest FXIII-B2 circulates bound to fibrinogen may reveal part of a stepwise mechanism that leads to production of fibrinogen/FXIII-A2B2 complexes. In addition, these data may have important implications for understanding the mechanism of action of therapeutic rFXIII-A2 infusion for FXIII-A deficiency. Binding of infused rFXIII-A2 to fibrinogen-bound FXIII-B2 (vs “free” FXIII-B2) ensures that rFXIII-A2 becomes incorporated into a functional, fibrinogen-bound complex that is crucial for normal FXIII activation and function.
Our study has potential limitations. First, loss of FXIII binding to Fibγ390-396A may reflect disrupted structure within the γ domain. However, this possibility seems unlikely because crystallographic studies suggest this region is disordered even in the native molecule.46-48 Moreover, although the pattern of fibrin crosslinking is subtly altered in both mouse4 and human Fibγ390-396A clots, both murine4,21 and human Fibγ390-396A can be fully crosslinked at the canonical residues located immediately C-terminal to γ390-396 (γQ398/399 and γK406). Thus, mutations within these residues do not appear to catastrophically alter structure within this domain. Second, the protocol used to precipitate fibrinogen may also promote FXIII precipitation and/or FXIII interaction with fibrinogen, and we and others8 have observed spontaneous precipitation of isolated FXIII-B subunits in certain experiments. However, glycine did not precipitate FXIII from afibrinogenemic plasma or in the presence of nonbinding Fibγ390-396A fibrinogen. Moreover, data from the precipitation experiments were supported by both SPR analyses and functional FXIII activation assays performed in the absence of glycine.
In summary, our data expose critical molecular interactions mediating FXIII binding to fibrinogen. Identification of these motifs advances our understanding of this interaction in both physiologic and pathophysiologic situations.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marvin T. Nieman and David C. Williams Jr for thoughtful suggestions, Susan T. Lord for the recombinant fibrinogen expression system, Feng-Chang Lin for assistance with statistical analysis, and Caroline Schuerger and LiFang Ping for excellent technical assistance.
This work was supported by funding from the National Institutes of Health (National Heart, Lung, and Blood Institute R56HL094740 and R01HL126974 [A.S.W.], R01HL112603 [M.J.F.], and National Center for Advancing Translational Sciences 1UL1TR001111 [UNC/A.S.W.]), a National Science Foundation Graduate Research Fellowship (DGE-1144081) (J.R.B.), and British Heart Foundation Project (PG/08/052/25172) and Special Project (SP/12/11/29786) grants (H.P.).
Authorship
Contribution: J.R.B. designed and performed experiments, analyzed and interpreted the data, and wrote the manuscript; C.W., A.M.B., and C.B.B. performed experiments and analyzed data; M.J.F. provided vital reagents; H.P. designed experiments and interpreted data; A.S.W. designed the research, analyzed and interpreted the data, and wrote the manuscript; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, University of North Carolina at Chapel Hill, 819 Brinkhous-Bullitt Building, CB #7525, Chapel Hill, NC 27599-7525; e-mail: alisa_wolberg@med.unc.edu.

![Figure 1. Fibrinogen residues γ390-396 are necessary for FXIII-A2B2 binding. Recombinant human fibrinogen variants (γA/γA, γ′/γ′, Aα251, and Fibγ390-396A) were mixed with FXIII-A2B2 (1 mg/mL [2.9 μM] and 20 µg/mL [60 nM], final, respectively) and precipitated with glycine. (A) Representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial sample (I), pellet (P), or supernatant (S). Note that P and S samples were prepared after the addition of glycine and are therefore diluted relative to the I sample. (B) Quantitation of all blots, indicating percent of FXIII-A or FXIII-B in the pellet, relative to total FXIII in the pellet and supernatant. Bars are means ± SEM; N = 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/15/10.1182_blood-2016-04-712323/4/m_1969f1.jpeg?Expires=1767705322&Signature=OekB~5etsWqV-mYXOpQGP6YgGsl05Fpk-azl6cIpkL-p4WsEpmZvj4k-FmNLfzJ2GzcyjScEifptl-agFZowxpHaQ-pD8CQZtV92hVKQIs~AKk~6nuVmsPkY422z7V~niB7vSGm1I6s6H2yDlnTIbMqqfkU5Z-VRRoqviorplOpDNQS9mqkZEnZKM33GmTdIuWSamOvJVTGRKsvmE77BXFoID24DX8Jk16KvKubbQiH973xt8t2gAFga~aonxJpXJ9zOuDnmiMgKZz-I-osHdrVAypG418uGY8iVj~vLJYFOOBHtlV7Rb9a4LOk4NqtATFMijOnpVeZzuOMp2fUuHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Fibrin(ogen) residues γ390-396 mediate the acceleratory effect of fibrin(ogen) on FXIII-A2B2 activation. FXIII-A2B2 (20 µg/mL [60 nM], final) was mixed with recombinant fibrinogens (γA/γA or Fibγ390-396A, 150 µg/mL [440 nM], final) or buffer (No Fgn). Reactions were triggered by addition of thrombin (2 nM, final) and CaCl2 (10 mM, final), quenched at the indicated time points, and analyzed by SDS-PAGE with western blotting and densitometry. Activation peptide cleavage was detected using anti-FXIII-A antibody. Fibrin crosslinking was detected using anti-fibrinogen antibody. (A) Representative western blots and (B) quantitation of FXIII-A2B2 activation over time from all blots. (C) Maximal rates of FXIII-A2B2 activation calculated from panel B. (D) Representative western blots of fibrin crosslinking, and quantitation of (E) γ-γ dimer formation and (F) γ-γ dimer formation rate. Data are means ± SEM; N = 3-6 replicates per time point.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/15/10.1182_blood-2016-04-712323/4/m_1969f2.jpeg?Expires=1767705322&Signature=DZrHmYOmFeGzHQP3oIqHqX74l79OCNenMBLzbO26EDgk0bf2jbaxCj9g0gQiEqiQR0plNj9j-evcmNLqL4Vo6nT2EOZA6u3BEgkEuAzCZOlyKJcImIgxx5KmZUSGDZqlteYFRWyJ6fb196Xlq4lU175fI~nsTyNAavXmjunSeb1Vnj89ugO69VGppfGKzL~hhgloVtJmYCnvpi-De3BgQmZ6T2lZMeMjQBm9vAmrbmWbez38vKMttMFGP5~oekrx9OXe-SObzmEfwhIf13hEqBvZ6AhX2ERIrHcaBq876-hw2qyNZNXc7qTGKfCsyHuDKjhaAYoHFTCpE9KE3AmMiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Fibrin(ogen) residues γ390-396 do not accelerate FXIII-A2 activation. rFXIII-A2 (10 µg/mL [60 nM], final) was mixed with γA/γA or Fibγ390-396A fibrinogen (150 µg/mL [440 nM], final). Reactions were triggered by addition of thrombin (2 nM, final) and CaCl2 (10 mM, final), quenched at the indicated time points, and analyzed by SDS-PAGE with western blotting and densitometry. Activation peptide cleavage was detected using anti-FXIII-A antibody. Fibrin crosslinking was detected using anti-fibrinogen antibody. (A) Representative western blots and (B) quantitation of rFXIII-A2 activation over time from all blots. (C) Maximal rates of rFXIII-A2 activation were calculated from quantified western blots. (D) Representative western blots of fibrin crosslinking and quantification of (E) γ-γ dimer formation and (F) formation rate. Data are means ± SEM; N = 4 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/15/10.1182_blood-2016-04-712323/4/m_1969f3.jpeg?Expires=1767705322&Signature=cWtUc21wkLU0E2JXTM1LJXBqLw2prIE8ODBrWToVNLXilmvLo5NpkUAbm5zEXZjFI0bcAcYNEwz3YhOfLt6~0eWVRAxdUH1Z6Vbz9CyuhaVcvsZqllwvu9~F0hXYArkdE3dQB9~OzGt1WSPn5ewVH2X-LBNd5fUlDOQQ7ux83I1iEm3-kXTLazfIGFDO0dtPEOY~hFTOGGT6rVieASupnLfaLg~CNyJFhVA7C5U0Y2IzCQhLJXZwVHBPv1EHTTY-nlbWZGVCJbilzLTHrRaTXX75w-bP5hBM0dTNd-veHmBDaI5-aNR4ckazL~dK-VmAgbjE~Q8K-N7P9IPMob3ygg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. FXIII-A2B2 binds fibrinogen residues γ390-396 via the FXIII-B subunits. rFXIII-A2 (10 µg/mL [60 nM], final), rFXIII-B2 (10 µg/mL [62 nM], final), or both, were mixed with (A) γA/γA or (B) Fibγ390-396A fibrinogen (1 mg/mL [2.9 μM], final) and precipitated with glycine. (A-B) Representative western blots for fibrinogen (Fgn), FXIII-A, and FXIII-B in the initial sample (I), pellet (P), or supernatant (S). Note that P and S samples were prepared after the addition of glycine and are therefore diluted relative to the I sample. Blots are representative of N = 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/15/10.1182_blood-2016-04-712323/4/m_1969f4.jpeg?Expires=1767705322&Signature=l69mxiVXhvsOtH4JJV~fUaP3BKBvm5t-Rwnenxeeeb5fAqML-OEC3wGVeUSeZwjh65dFUJWzfgew-NSscIot8Z6fiIii3MoXV~CyyHxJx~hCMDYb~2B0P32A4TNl~8K2XJC7nIHcOd5qIFXidHNDJW5cXRISVJsRCrJN5ncnBnq7f63Ku09t-6Vj21hEEcVUOHyFtroNLKwahalLPh4nenQxZ5hFeJCdoWWK10rJPnsE3gI82o9QKR0YC9Q7eupIkHYLyjivAwphqFTDjwFzw~iJ~qP8O3ZY58aYed3zRRoNoa-WRMc5tAUa0UVtAXGtuAMFD83R6vV2M19-9-3YZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)