Key Points
The A1 domain of VWF contains a cryptic binding site that plays a key role in regulating macrophage binding and clearance.
The N-linked glycans presented at N1515 and N1574 within the A2 domain of VWF modulate macrophage-mediated clearance.
Abstract
Enhanced von Willebrand factor (VWF) clearance is important in the etiology of von Willebrand disease. However, the molecular mechanisms underlying VWF clearance remain poorly understood. In this study, we investigated the role of VWF domains and specific glycan moieties in regulating in vivo clearance. Our findings demonstrate that the A1 domain of VWF contains a receptor-recognition site that plays a key role in regulating the interaction of VWF with macrophages. In A1-A2-A3 and full-length VWF, this macrophage-binding site is cryptic but becomes exposed following exposure to shear or ristocetin. Previous studies have demonstrated that the N-linked glycans within the A2 domain play an important role in modulating susceptibility to ADAMTS13 proteolysis. We further demonstrate that these glycans presented at N1515 and N1574 also play a critical role in protecting VWF against macrophage binding and clearance. Indeed, loss of the N-glycan at N1515 resulted in markedly enhanced VWF clearance that was significantly faster than that observed with any previously described VWF mutations. In addition, A1-A2-A3 fragments containing the N1515Q or N1574Q substitutions also demonstrated significantly enhanced clearance. Importantly, clodronate-induced macrophage depletion significantly attenuated the increased clearance observed with N1515Q and N1574Q in both full-length VWF and A1-A2-A3. Finally, we further demonstrate that loss of these N-linked glycans does not enhance clearance in VWF in the presence of a structurally constrained A2 domain. Collectively, these novel findings support the hypothesis that conformation of the VWF A domains plays a critical role in modulating macrophage-mediated clearance of VWF in vivo.
Introduction
Von Willebrand factor (VWF) is a large multimeric sialoglycoprotein that plays 2 key roles in normal hemostasis.1,2 First, it mediates recruitment of platelets following injury to the vascular endothelium. Second, VWF also functions as a carrier molecule for factor VIII. In vivo expression of VWF occurs only within endothelial cells (ECs)3 and megakaryocytes,4 where VWF is initially synthesized as a monomer composed of a series of repeating domains in the order D′-D3-A1-A2-A3-D4-C1-C2-C3-C4-C5-C6-CK. VWF synthesized within ECs undergoes constitutive secretion into the plasma. Prior to this secretion, VWF undergoes complex posttranslational modification within ECs, including significant N- and O-linked glycosylation.2,5,6
The N- and O-linked glycans of human VWF have both been characterized and demonstrate significant heterogeneity.7,8 Monosialylated or disialylated biantennary complex-type chains constitute the most common N-linked glycans expressed on VWF.6,7,9-11 Although the O-linked glycans of VWF also demonstrate marked heterogeneity, disialyl core 1 structures account for ∼70% of the total population.8,12 Importantly, although the majority of its glycans are capped by negatively charged sialic acid,7,13 VWF is unusual in that a minority of both N-linked and O-linked carbohydrate chains express terminal ABO(H) blood group determinants.7,8,11
Although substantial progress has been achieved in understanding the biosynthesis, structure, and functions of VWF, the biological mechanism(s) responsible for modulating VWF clearance from the plasma remain poorly understood.14 Nevertheless, accumulating data have shown that enhanced VWF clearance plays an important role in the etiology of both type 1 and type 2 VWD.15-20 Recent evidence further suggests that hepatic and splenic macrophages may play key roles in mediating VWF clearance.21-26 For example, differentiated primary human macrophages can bind and endocytose purified VWF in vitro.21 Furthermore, macrophage depletion significantly prolonged the in vivo survival of VWF infused into VWF−/− mice.21,24,25 In addition, the macrophage lipoprotein receptor (LRP1) has been shown to bind to VWF in a shear-dependent manner.23 Critically however, the specific regions of the VWF glycoprotein involved in modulating macrophage interactions remain unclear.
More than 30 different VWF point mutations have already been associated with enhanced clearance in patients with VWD.15,27,28 Intriguingly, the majority of VWF amino acid substitutions associated with enhanced clearance are clustered within the A1 domain.14 Emerging evidence suggests that at least some of these VWF mutations result in enhanced macrophage-mediated clearance in vivo. For example, Wohner et al recently showed that specific type 2B VWD variants (R1306Q and V1316M within the A1 domain) result in increased VWF clearance that is predominantly modulated through the macrophage LRP1 receptor.26 Nevertheless, the molecular mechanism through which so many different amino acid substitutions clustered within the A1 domain of VWF result in enhanced clearance remains poorly understood.
Variation in VWF glycosylation profile has also been shown to significantly influence the clearance rate.29-34 For example, terminal ABO(H) blood group determinants significantly modulate in vivo clearance.35 Consequently, plasma VWF levels are significantly lower in blood group O individuals than in non-O individuals.5,36 The asialoglycoprotein or Ashwell receptor (ASGPR) is a C-type lectin predominantly expressed on hepatocytes and is composed of 2 transmembrane protein subunits (Asgr-1 and Asgr-2).37 Importantly, a role for Asgr1 in modulating VWF clearance has recently been described.34 In addition, a number of other carbohydrate receptors, including galectin-1 (Gal-1), galectin-3 (Gal-3), Siglec-5, and CLEC4M, have also been shown to bind VWF.38-40 Furthermore, genome-wide association studies have reported associations between some of these receptors and plasma levels of the VWF–factor VIII complex.41 Nevertheless, given the complexity and heterogeneity of the N- and O-linked glycans expressed on VWF, the biological mechanisms through which specific VWF carbohydrate structures serve to regulate in vivo clearance remain poorly defined. In this study, we have used a series of in vitro and in vivo methodologies to investigate the importance of specific domains and glycans in modulating VWF clearance. Our findings demonstrate that N-linked glycans at N1515 and N1574 within the A2 domain of VWF play a critical role in protecting VWF against macrophage-mediated clearance.
Materials and methods
Expression and purification of recombinant VWF
The expression vector pcDNA-VWF encoding full-length recombinant VWF (rVWF) has previously been described.42 A DNA fragment containing VWFA1A2A3 (residues 1260-1874) was inserted into expression vector pEXPR-IBA 42 (IBA) via NheI and PmeI restriction sites. The same strategy was used for expressing VWFA1 (residues 1239-1472), VWFA2 (residues 1473-1668), and VWFA3 (residues 1671-1878). Human full-length rVWF, A1A2A3-VWF A1-VWF, A2-VWF, and A3-VWF were transiently expressed in HEK293T cells. Conditioned serum-free medium was harvested and concentrated as before.24 A1-VWF, A2-VWF, A3-VWF, and A1A2A3-VWF variants were further purified via nickel affinity chromatography.
Site-directed mutagenesis of VWF was carried out to introduce point mutations at N1515 and N1574 within both full-length and truncated A1A2A3-VWF. In keeping with previous studies defining the biological significance of these glycans,42-44 each asparagine residue was mutated to glutamine (N1515Q and N1574Q) to eliminate the N-linked glycans at these positions. Mutations were verified by DNA sequencing to ensure the absence of any other randomly introduced mutations. Similarly, mutagenesis of full-length VWF was carried out to introduce point mutations N1493C and C1670S. These mutations result in the creation of a homologous disulfide bond in the A2 domain of VWF between the cysteine at 1493 and the native cysteine at 1669. This cysteine clamp mutation has previously been described to prevent A2 domain unfolding and render VWF insensitive to ADAMTS13 cleavage.45,46
In vitro modification of VWF glycan structures
The N-linked glycan profile of rVWF and A1A2A3-VWF was modified using a specific exoglycosidase peptide N glycosidase F (PNGase F; New England Biolabs). VWF glycan digestions were carried out overnight under nondenaturing conditions at 37°C as previously reported.42 After digestion, residual VWF glycan expression was quantified using specific lectin enzyme-linked immunosorbent assays (ELISAs) as described previously.47,48
Human RAP expression and purification
Low-density lipoprotein receptor-related protein-associated protein 1 (RAP) acts as a molecular chaperone by inhibiting ligand binding to LRP1, as well as other members of this receptor family. Human RAP complementary DNA coding Tyr35 to Leu357 (UNIPROTKB-P30533) was inserted into Novagen pET28a(+) bacterial expression vector (Novagen, Nottingham, UK) via BamHI and SalI restriction sites. Recombinant RAP protein was refolded from Escherichia coli inclusion bodies as described previously.49
THP-1 binding assay
THP-1 cells were seeded on microwell plates (Nunclon; Fisher Scientific) at a density of 5 × 106 cells/mL. For differentiation, media was supplemented with 20 ng/mL PMA (Sigma-Aldrich). After 72 hours, fresh growth medium was added to the cells, which were then rested for an additional 4 days before use.50 VWF-THP-1 binding was performed at 4°C to prevent endocytosis. Full-length VWF or truncated A1A2A3-VWF variants were diluted in ice-cold serum-free growth medium incubated with the cells for 1 hour on ice. For analysis, the nuclei were stained with Hoechst 33342 (Thermo Fisher). Full-length bound VWF was detected using anti–human VWF (Dako) followed by Alexa Fluor 488 donkey anti–rabbit immunoglobulin G (IgG) (Life Technologies). Truncated A1A2A3-VWF variants were detected by Penta-His Alexa Fluor 488 conjugate (QIAGEN). THP-1 surface-bound VWF was quantified using the fluorescence microscopy IN Cell Analyzer 1000 (GE Healthcare). Eight fields of view were imaged per well at a magnification of ×20. Image analysis was carried out using high throughput IN Cell 1000 Image Analysis Software (GE Healthcare). Data were graphed as percentage fluorescently labeled VWF per cell relative to maximal VWF binding (mean ± standard error of the mean [SEM]).
VWF uptake by differentiated THP-1 cells was assessed using confocal microscopy. In brief, cells were seeded onto glass chamber sides and differentiated using PMA as above. Subsequently cells were incubated with VWF or glycoforms thereof in the presence of ristocetin (1 mg/mL) for 30 minutes at either 4°C or 37°C to assess binding and internalization, respectively. Cells were fixed with 4% paraformaldehyde for 20 minutes, in some cases permeabilized using 0.1% Triton X, and then incubated with 4,6-diamidino-2-phenylindole; polyclonal mouse anti-EEA1 (early endosomal antigen-1) (BD Biosciences); and/or polyclonal rabbit anti-VWF for 45 minutes. After washing, slides were stained with Alexa Flour 594–conjugated anti-mouse IgG (Invitrogen) and Alexa Flour 488–conjugated anti–rabbit IgG (Invitrogen, UK) for 15 minutes. Images were visualized using LSM700 (Carl Zeiss) Confocal Microscope, 63× plan-apochromat lens. Images were analyzed using ImageJ and Adobe Illustrator CC2015.3.
VWF clearance studies in VWF−/− mice
VWF−/− mice were obtained from The Jackson Laboratory (Sacramento, CA). All animal experiments were approved by the Animal Research Ethics Committee, Trinity College Dublin, and were performed in compliance with the Irish Medicines Board regulations. VWF clearance studies were performed using mice between 6 and 10 weeks of age. In brief, VWF−/− mice were injected intravenously with 30 nM (37.5 U/kg) of VWF or glycoforms thereof. At sequential time points, blood was collected into heparin-coated micro containers. Three to five mice per time point were used. Residual plasma VWF:antigen levels were determined by ELISA. In vivo macrophage depletion was performed as previously described,24,51 using IV infusion of clodronate liposomes (100 µl/10 g body weight). Control mice were injected with PBS liposomes. To examine the role of the LRP1 clearance receptor, mice were administered 200 µM LRP1-antagonist RAP 1 minute prior to injection of VWF.
Data presentation and statistical analysis
All experimental data and statistical analysis were performed using the GraphPad Prism program. Data are expressed as mean ± SEM. To assess statistical differences for 2 data sets, data were analyzed using the Student unpaired 2-tailed t test. For multiple comparisons, data were analyzed using a one-way analysis of variance with post hoc Dunnett’s test. For all statistical tests, P < .05 was considered significant.
Results
The A domains of VWF modulate macrophage-mediated clearance
Lenting et al previously reported that a truncated A1-A2-A3 VWF fragment expressed in BHK cells demonstrated a similar clearance pattern to that of full-length rVWF and postulated that a receptor-recognition site may be present within the A1-A3 region.52 To further study this hypothesis, we first investigated the clearance of rVWF and A1-A2-A3 that was expressed in a different cell line (HEK293T). Following tail vein injection in VWF−/− mice, A1-A2-A3 (HEK) clearance was also similar to that of full-length VWF (HEK) (Figure 1A). Recent studies have shown that hepatic macrophages contribute to the clearance of full-length VWF.21-26 To determine whether macrophages modulate A1-A2-A3 clearance in vivo, clearance studies were repeated following clodronate-induced macrophage depletion. In keeping with previous studies, clearance of full-length rVWF was significantly reduced following macrophage depletion (Figure 1B). Interestingly, in vivo clearance of A1-A2-A3 was also significantly attenuated following macrophage depletion (Figure 1B).
The A domains of VWF modulate macrophage-mediated clearance. (A) The in vivo clearance of a monomeric A1-A2-A3 VWF fragment in VWF−/− mice was compared with that of full-length rVWF. At each time point, the residual circulating VWF concentration was determined by VWF:antigen ELISA. All results are plotted as percentage residual VWF levels relative to the amount injected. Data are presented as mean ± SEM. In some cases, the SEM cannot be seen due to its small size. Mean residence times for full-length and A1-A2-A3 were 11.3 ± 0.6 and 10.0 ± 0.61 minutes, respectively. (B) To study the role of macrophages in modulating clearance of A1-A2-A3 and full-length rVWF, in vivo clearance studies were repeated in VWF−/− mice 24 hours following clodronate-induced macrophage depletion. Blood was collected at 3- and 10-minute time points, and residual VWF quantified by ELISA. (C) The in vitro binding of A1-A2-A3 to macrophages was assessed using THP-1 macrophage cells as detailed in “Materials and methods.” (D) Individual A-domain proteins A1, A2, and A3 were examined for binding to THP-1 macrophages. Significant binding was observed for the A1 domain compared with the A2 and A3 domains (*P < .05, **P < .01, and ***P < .001, respectively; negative control is no VWF). (E) To investigate the role of VWF carbohydrate determinants in modulating VWF clearance, rVWF was treated with PNGase F (PNG-rVWF). N-linked glycan removal was confirmed using a specific lectin ELISA. In vivo survival was then measured in VWF−/− mice as before. Results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are represented as mean ± SEM. (F) To assess a potential role for VWF N-linked glycans in the A domains in regulating macrophage binding, A1-A2-A3 was treated with PNGase to remove the N-linked glycans in the A2 domain at N1515 and N1574 (PNG-A1A2A3). The ability of PNG-A1A2A3 to bind to THP-1 macrophages in the presence of ristocetin was then compared with WT A1-A2-A3 using HiContent image analysis as before. Data are graphed as percentage binding relative to maximal (mean ± SEM) (****P < .001).
The A domains of VWF modulate macrophage-mediated clearance. (A) The in vivo clearance of a monomeric A1-A2-A3 VWF fragment in VWF−/− mice was compared with that of full-length rVWF. At each time point, the residual circulating VWF concentration was determined by VWF:antigen ELISA. All results are plotted as percentage residual VWF levels relative to the amount injected. Data are presented as mean ± SEM. In some cases, the SEM cannot be seen due to its small size. Mean residence times for full-length and A1-A2-A3 were 11.3 ± 0.6 and 10.0 ± 0.61 minutes, respectively. (B) To study the role of macrophages in modulating clearance of A1-A2-A3 and full-length rVWF, in vivo clearance studies were repeated in VWF−/− mice 24 hours following clodronate-induced macrophage depletion. Blood was collected at 3- and 10-minute time points, and residual VWF quantified by ELISA. (C) The in vitro binding of A1-A2-A3 to macrophages was assessed using THP-1 macrophage cells as detailed in “Materials and methods.” (D) Individual A-domain proteins A1, A2, and A3 were examined for binding to THP-1 macrophages. Significant binding was observed for the A1 domain compared with the A2 and A3 domains (*P < .05, **P < .01, and ***P < .001, respectively; negative control is no VWF). (E) To investigate the role of VWF carbohydrate determinants in modulating VWF clearance, rVWF was treated with PNGase F (PNG-rVWF). N-linked glycan removal was confirmed using a specific lectin ELISA. In vivo survival was then measured in VWF−/− mice as before. Results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are represented as mean ± SEM. (F) To assess a potential role for VWF N-linked glycans in the A domains in regulating macrophage binding, A1-A2-A3 was treated with PNGase to remove the N-linked glycans in the A2 domain at N1515 and N1574 (PNG-A1A2A3). The ability of PNG-A1A2A3 to bind to THP-1 macrophages in the presence of ristocetin was then compared with WT A1-A2-A3 using HiContent image analysis as before. Data are graphed as percentage binding relative to maximal (mean ± SEM) (****P < .001).
To further investigate a putative role for the A domains in regulating VWF clearance by macrophages, VWF binding to PMA-differentiated THP-1 cells in vitro was studied using HiContent image analysis. In preliminary studies, we observed that full-length plasma-derived VWF (pd-VWF) and rVWF both bound to THP-1 macrophages (supplemental Figure 1, available on the Blood Web site). In keeping with a role for the A domains in modulating macrophage interaction, full-length VWF binding was significantly enhanced in the presence of ristocetin (1 mg/mL) (supplemental Figure 1). Moreover, dose-dependent binding of the truncated A1-A2-A3 fragment to THP-1 macrophages in vitro was also observed (Figure 1C). Finally, the relative importance of the individual A domains within the A1-A2-A3 construct in determining macrophage binding was investigated. Significant in vitro binding of the A1 domain to THP-1 macrophages was observed (Figure 1D). However, in contrast, no significant binding was seen for the isolated A2 or A3 domains. Importantly, the macrophage binding of the isolated A1 domain was also significantly greater than that of the combined A1-A2-A3 fragment. Collectively, these data support the hypothesis that the A domains of VWF contain a receptor-recognition site important in regulating the interaction of VWF with macrophages and suggest that the VWF A1 domain plays a particular critical role in determining macrophage binding.
In previous studies, we observed that PNGase F digestion of human pd-VWF to remove the N-linked glycans resulted in markedly increased clearance. Similarly, clearance of rVWF was also significantly increased ∼2.5-fold following PNGase digestion (P < .05) (Figure 1E). Given the important role of A1-A2-A3 in regulating VWF clearance by macrophages, it is interesting that only 2 N-linked glycan sites are located in this region (at N1515 and N1574 within the A2 domain).7,42 To determine whether these N-linked glycans within A2 play a role in modulating VWF interaction with macrophages, binding of A1-A2-A3 before and after PNGase F digestion was investigated. Following PNGase treatment, in vitro binding of A1-A2-A3 to THP-1 macrophages was markedly increased (Figure 1F), suggesting that these N-linked glycans within A2 play a novel role in regulating macrophage interaction.
N-linked glycans at N1515 and N1574 are critical determinants of VWF clearance
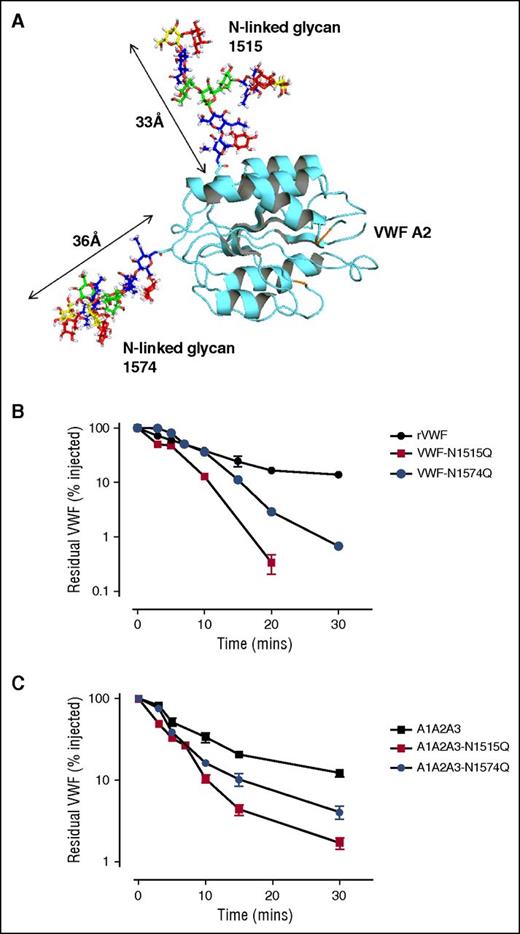
Recent mass spectrometry analysis has demonstrated that both of the N-linked glycans at N1515 and N1574 within A2 are occupied by large complex carbohydrate structures (Figure 2A).7 To further investigate the role of these glycans in modulating macrophage interaction, the asparagine residues at N1515 and N1574 were individually mutated to glutamine residues (VWF-N1515Q and VWF-N1574Q) and expressed in HEK293T cells. In accordance with previous studies,42 VWF-N1515Q and VWF-N1574Q both displayed multimer distribution and collagen binding activities similar to wild-type (WT) VWF (data not shown). Following tail vein injection, in vivo clearance of VWF-N1515Q and VWF-N1574Q was markedly enhanced in VWF mice compared with WT-VWF (Figure 2B). Interestingly, clearance of VWF-N1515Q was faster than that of any other previously described VWF point mutations, including VWF-R1205H (data not shown; P < .05).24 In keeping with previous studies, infusion of VWF-N1515Q and VWF-N1574Q had no significant effect on murine platelet counts (data not shown).42 In addition, SDS-PAGE of murine plasma samples under reducing conditions demonstrated no significant evidence of in vivo proteolysis of either VWF-N1515Q or VWF-N1574Q.
N-linked glycans at N1515 and N1574 are critical determinants of VWF clearance in vivo. (A) A model of the VWF A2 domain was prepared as previously described.66 Mass spectrometry analysis of human pd-VWF has provided extensive information regarding the N-glycome of VWF. Utilizing this information, a model of the VWF A2 domain with its associated glycans was constructed using Glycam Glycoprotein Builder software. N1515 and N1574 glycans structures were mapped onto the A2 domain crystal structure using this glycan modeling. This in silico analysis revealed that the complex glycans at N1515 and N1574 were both of significant size, spanning ∼33 Å and ∼36 Å in length, respectively. (B) To investigate a potential role for specific glycan sites in influencing VWF clearance, N1515 and N1574 in the A2 domain were targeted for removal by site-directed mutagenesis (VWF-N1515Q and VWF-N1574Q, respectively). In vivo clearance studies of these VWF glycan variants were performed as before and compared with WT rVWF. (C) Given that the glycans N1515 and N1574 reside within the A2 domain of VWF, we further sought to examine if these glycans could also influence the in vivo survival of an A1A2A3 VWF truncated fragment. To this end, site-directed mutagenesis was performed to eliminate the glycan at N1515 (A1A2A3-N1515Q) and N1574 (A1A2A3-N1574Q). Clearance examined in VWF−/− mice as before. All results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are presented as mean ± SEM.
N-linked glycans at N1515 and N1574 are critical determinants of VWF clearance in vivo. (A) A model of the VWF A2 domain was prepared as previously described.66 Mass spectrometry analysis of human pd-VWF has provided extensive information regarding the N-glycome of VWF. Utilizing this information, a model of the VWF A2 domain with its associated glycans was constructed using Glycam Glycoprotein Builder software. N1515 and N1574 glycans structures were mapped onto the A2 domain crystal structure using this glycan modeling. This in silico analysis revealed that the complex glycans at N1515 and N1574 were both of significant size, spanning ∼33 Å and ∼36 Å in length, respectively. (B) To investigate a potential role for specific glycan sites in influencing VWF clearance, N1515 and N1574 in the A2 domain were targeted for removal by site-directed mutagenesis (VWF-N1515Q and VWF-N1574Q, respectively). In vivo clearance studies of these VWF glycan variants were performed as before and compared with WT rVWF. (C) Given that the glycans N1515 and N1574 reside within the A2 domain of VWF, we further sought to examine if these glycans could also influence the in vivo survival of an A1A2A3 VWF truncated fragment. To this end, site-directed mutagenesis was performed to eliminate the glycan at N1515 (A1A2A3-N1515Q) and N1574 (A1A2A3-N1574Q). Clearance examined in VWF−/− mice as before. All results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are presented as mean ± SEM.
To investigate the molecular mechanism responsible for the enhanced VWF clearance associated with the loss of A2 domain N-linked glycans, we further characterized the effects of introducing the VWF-N1515Q and VWF-N1574Q substitutions into the A2 domain of the A1-A2-A3 fragment (A1-A2-A3-N1515Q and A1-A2-A3-N1574Q). In keeping with their effect in reducing the survival of full-length rVWF, both mutations also resulted in significantly enhanced clearance of the A1-A2-A3 fragment (Figure 2C). Cumulatively, these novel findings demonstrate that both of the N-linked glycans located at N1515 and N1574 within the A2 domain of VWF play critical roles in regulating clearance in vivo. Furthermore, the increased clearance associated with loss of N-linked glycan structures at either N1515 or N1574 is modulated through local effects within the A1-A2-A3 region.
N-linked glycans within A2 regulate enhanced clearance
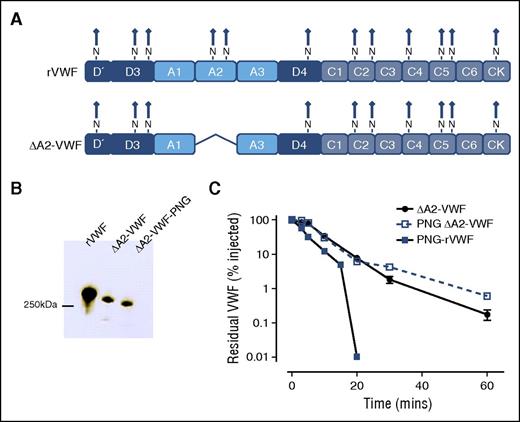
Besides the 2 N-linked glycans located within the A2 domain, another 10 N-linked glycans are present on the VWF monomer (Figure 3A).7,43 To determine whether these additional N-linked glycans may also be important in regulating VWF clearance, we expressed a rVWF variant from which the A2 domain was deleted (ΔA2-VWF) (Figures 3A-B). Interestingly, in vivo clearance of ΔA2-VWF was not accelerated following PNGase F digestion (Figure 3C), suggesting that the N-linked glycans within the A2 domain play a critical role in modulating the enhanced clearance of PNG-VWF.
N-linked glycans within the VWF A2 domain regulate enhanced clearance. Our findings suggest that the N-linked glycan within A2 may have a specific role in modulating VWF clearance. To examine if glycans outside the A2 domain may also influence VWF survival, a fragment of VWF with the A2 domain deleted was constructed. (A) Consequently, this VWF variant (ΔA2-VWF) fails to express the N-linked glycans N1515 and N1574. (B) This VWF variant was subjected to PNGase F treatment (PNG ΔA2-VWF) to remove all remaining N-linked glycans. (C) Clearance was assessed in VWF−/− mice as before. All results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are presented as mean ± SEM.
N-linked glycans within the VWF A2 domain regulate enhanced clearance. Our findings suggest that the N-linked glycan within A2 may have a specific role in modulating VWF clearance. To examine if glycans outside the A2 domain may also influence VWF survival, a fragment of VWF with the A2 domain deleted was constructed. (A) Consequently, this VWF variant (ΔA2-VWF) fails to express the N-linked glycans N1515 and N1574. (B) This VWF variant was subjected to PNGase F treatment (PNG ΔA2-VWF) to remove all remaining N-linked glycans. (C) Clearance was assessed in VWF−/− mice as before. All results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are presented as mean ± SEM.
Accelerated clearance of VWF N1515Q and VWF N1574Q is mediated by macrophages
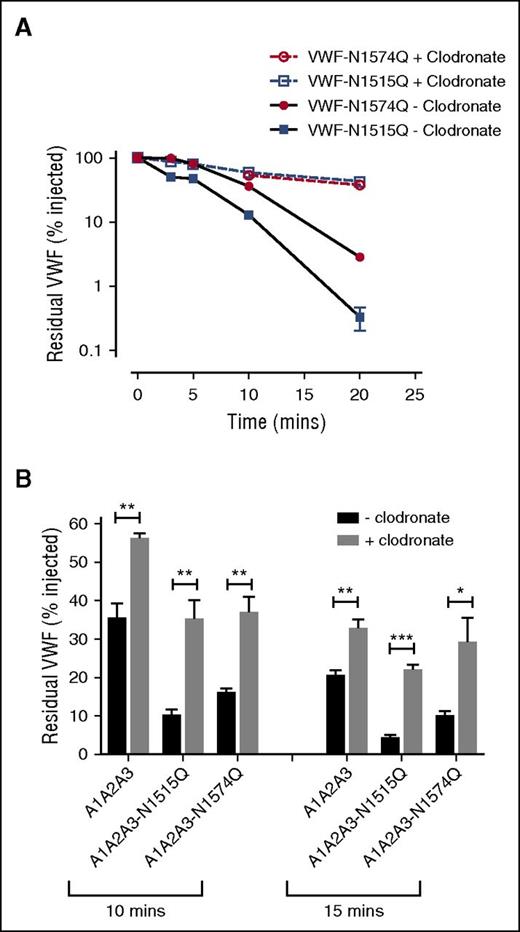
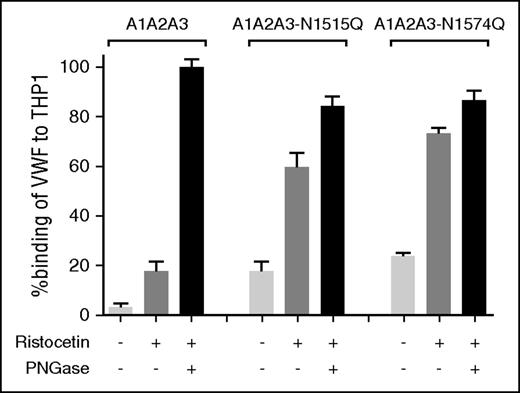
To investigate whether VWF A2 domain glycan expression influences macrophage-mediated clearance, studies were repeated in VWF−/− mice following clodronate administration. We found that the increased clearance phenotypes of both VWF-N1515Q and VWF-N1574Q were significantly attenuated following clodronate-induced macrophage depletion (Figure 4A). Similarly, the enhanced clearance of both A1-A2-A3-N1515Q and A1-A2-A3-N1574Q was also significantly reduced following macrophage depletion (Figure 4B). In keeping with previous reports,21,22 confocal microscopy studies performed following incubation at 4°C demonstrated that VWF-WT, VWF-N1515Q, and VWF-N1574Q all bound to differentiated THP-1 cells in vitro (supplemental Figure 2). When incubation studies were performed at 37°C, confocal studies showed colocalization of VWF-WT, VWF-N1515Q, and VWF-N1574Q with early endosomes (supplemental Figure 2), demonstrating that both VWF-N1515Q and VWF-N1574Q are taken up by macrophages. To further study the molecular mechanism through which the N-linked glycans expressed at N1515 and N1574 influence macrophage-dependent clearance, we used HiContent image analysis to compare the in vitro binding of A1-A2-A3-N1515Q, A1-A2-A3-N1574Q, and WT A1-A2-A3 to THP-1 cells. WT A1-A2-A3 demonstrated minimal macrophage binding (Figure 5). However, this binding was significantly enhanced in the presence of ristocetin (1 mg/mL). Importantly, following PNGase digestion to remove the 2 N-linked glycans at N1515 and N1574, binding of A1-A2-A3 was dramatically increased, suggesting that the presence of these carbohydrate structures serves to prevent binding of A1-A2-A3 to macrophages. In keeping with this hypothesis, both A1-A2-A3-N1515Q and A1-A2-A3-N1574Q demonstrated significantly increased binding compared with WT A1-A2-A3 in either the presence or absence of ristocetin. Finally, PNGase treatment of both A1-A2-A3-N1515Q and A1-A2-A3-N1574Q served to further enhance macrophage binding, confirming that both N-linked glycans contribute to modulating macrophage interaction. Cumulatively, these in vivo and in vitro data confirm that the N-linked glycans within the A2 domain of play important roles in regulating macrophage-dependent VWF clearance
Accelerated clearance of VWF N1515Q and VWF N1574Q is mediated by macrophages. (A) In order to assess the potential contribution of macrophages in modulating the enhanced clearance of VWF glycan variants, clearance of VWF N1515Q and VWF N1574Q was repeated in VWF−/− mice 24 hours after clodronate-induced macrophage depletion. (B) To determine whether macrophages play a role in regulating the reduced survival of A1A2A3-N1515Q and A1A2A3-N1574Q, in vivo clearance studies were also re-assessed in VWF−/− mice following clodronate treatment. Data are graphed as percentage residual VWF relative to the amount injection (*P < .05, **P < .01, and ***P < .001).
Accelerated clearance of VWF N1515Q and VWF N1574Q is mediated by macrophages. (A) In order to assess the potential contribution of macrophages in modulating the enhanced clearance of VWF glycan variants, clearance of VWF N1515Q and VWF N1574Q was repeated in VWF−/− mice 24 hours after clodronate-induced macrophage depletion. (B) To determine whether macrophages play a role in regulating the reduced survival of A1A2A3-N1515Q and A1A2A3-N1574Q, in vivo clearance studies were also re-assessed in VWF−/− mice following clodronate treatment. Data are graphed as percentage residual VWF relative to the amount injection (*P < .05, **P < .01, and ***P < .001).
N-linked glycans N1515 and N1574 modulate in vitro binding of VWF to macrophages. To examine the biological mechanisms mediating the enhanced clearance of A1A2A3-N1515Q/N1574Q, we assessed binding to THP-1 macrophages in vitro. The binding of A1A2A3 VWF and the glycan variants A1A2A3-N1515Q and A1A2A3-N1574Q to THP-1 macrophages was examined in the presence or absence of 1mg/ml ristocetin. Additionally, all the A1A2A3 variants were subjected to PNGase treatment to remove both N-linked glycans (black columns) and THP-1 macrophage binding was measured. Data are graphed as percentage binding relative to maximal binding (mean ± SEM).
N-linked glycans N1515 and N1574 modulate in vitro binding of VWF to macrophages. To examine the biological mechanisms mediating the enhanced clearance of A1A2A3-N1515Q/N1574Q, we assessed binding to THP-1 macrophages in vitro. The binding of A1A2A3 VWF and the glycan variants A1A2A3-N1515Q and A1A2A3-N1574Q to THP-1 macrophages was examined in the presence or absence of 1mg/ml ristocetin. Additionally, all the A1A2A3 variants were subjected to PNGase treatment to remove both N-linked glycans (black columns) and THP-1 macrophage binding was measured. Data are graphed as percentage binding relative to maximal binding (mean ± SEM).
Glycan structures at N1515 and N1574 in the A2 domain influence LRP1-mediated clearance
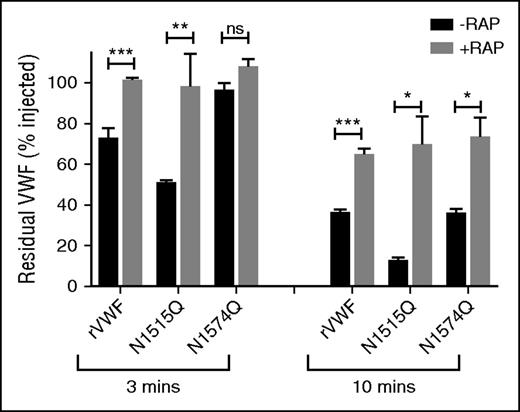
Recent studies have demonstrated that macrophage LRP1 plays an important role in regulating VWF clearance.23,26,53 In addition, LRP1 has been shown to bind to VWF in a shear-dependent manner. Importantly, Rastegarlari et al previously demonstrated that the inhibitory effects of RAP on VWF clearance were predominantly modulated through macrophage LRP1 rather than LRP1 expressed in other cells or other macrophage lipoprotein receptors.23 To further investigate whether VWF A2 domain glycans influence LRP1-mediated clearance in vivo, VWF-N1515Q and VWF-N1574Q clearance studies in VWF−/− mice were repeated in the presence or absence of RAP. In keeping with previous reports, we confirmed that clearance of WT-VWF was significantly reduced in the presence of RAP (Figure 6). Interestingly, the increased clearance of VWF-N1515Q and VWF-N1574Q were also both significantly attenuated in the presence of RAP (Figure 6). These findings are consistent with those observed above following clodronate-induced macrophage depletion (Figure 4A-B) and suggest that the VWF N-linked glycans at N1515 and N1574 modulate macrophage-dependent clearance at least in part through an LRP1-mediated mechanism.
Glycan structures at N1515 and N1574 in the A2 domain influence LRP1-mediated clearance. Recent studies have shown that macrophage LRP1 plays an important role in regulating in vivo clearance of VWF. Moreover, RAP prolongs VWF survival in vivo predominantly by inhibiting this macrophage LRP1 mediated clearance. To investigate whether the effect of VWF glycans on macrophage-mediated clearance were modulated via LRP1, clearance studies for wild type rVWF and glycan variants N1515Q and N1574Q were repeated in VWF−/− mice in the presence or absence of the LRP1 antagonist RAP. Blood was collected at 3 and 10 minutes after injection, and data are graphed as percentage residual VWF relative to the amount injected (*P < .05, **P < .01, and ***P < .001; ns, not significant).
Glycan structures at N1515 and N1574 in the A2 domain influence LRP1-mediated clearance. Recent studies have shown that macrophage LRP1 plays an important role in regulating in vivo clearance of VWF. Moreover, RAP prolongs VWF survival in vivo predominantly by inhibiting this macrophage LRP1 mediated clearance. To investigate whether the effect of VWF glycans on macrophage-mediated clearance were modulated via LRP1, clearance studies for wild type rVWF and glycan variants N1515Q and N1574Q were repeated in VWF−/− mice in the presence or absence of the LRP1 antagonist RAP. Blood was collected at 3 and 10 minutes after injection, and data are graphed as percentage residual VWF relative to the amount injected (*P < .05, **P < .01, and ***P < .001; ns, not significant).
Removal of the N-linked glycan at N1515 does not enhance clearance in VWF with a structurally constrained A2 domain
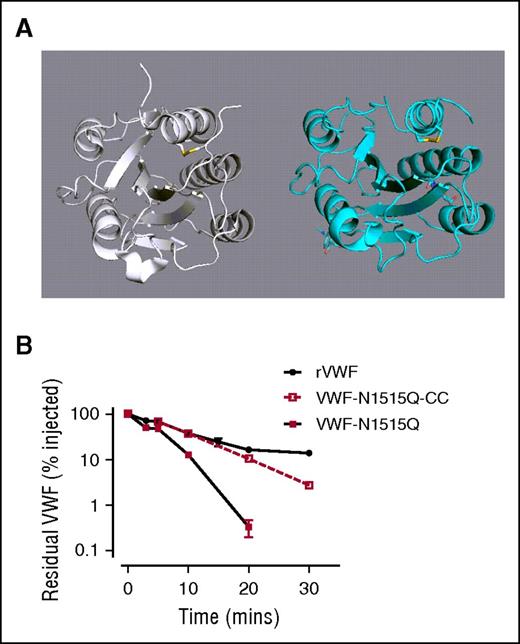
Large complex N-linked glycans, such as those expressed in the VWF A2 domain, have been previously shown to have important effects on glycoprotein conformation.54-57 We hypothesized that loss of A2 domain N-linked glycans causes conformational changes that result in enhanced VWF clearance by macrophages. Interestingly, recent studies have described a structurally constrained VWF variant with an engineered long-range disulfide bond (Cys1493-Cys1669) within the A2 domain (Figure 7A).45 To address our hypothesis, we therefore proceeded to express the N1515Q mutation in this cysteine-clamp VWF variant (VWF-CC-N1515). Insertion of the cysteine clamp alone in A2 (VWF-CC) had no significant effect upon A1-A2-A3 clearance compared with WT A1-A2-A3 (Figure 7B). Importantly however, the rapid in vivo clearance of A1-A2-A3 associated with loss of the N1515 glycan was ablated in the presence of this structurally constrained A2 domain (Figure 7B). Collectively, these data suggest that loss of the N-linked glycans in A2 trigger enhanced VWF clearance by macrophages through conformational changes.
Removal of the N-linked glycans at N1515 does not enhance clearance in VWF with a structurally constrained A2 domain. (A) To examine a potential role for A2 domain conformation in modulating clearance VWF, a previously described cysteine-clamp mutation (N1493C/C1670S) was inserted into full-length rVWF (rVWF-CC) and VWF-N1515Q (VWF-N1515Q-CC). This mutation creates a structurally constrained A2 due to the presence of a long-range disulfide bridge, homologous to those present in the A1 and A3 domains. (B) Clearance was assessed in VWF−/− mice. All results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are presented as mean ± SEM.
Removal of the N-linked glycans at N1515 does not enhance clearance in VWF with a structurally constrained A2 domain. (A) To examine a potential role for A2 domain conformation in modulating clearance VWF, a previously described cysteine-clamp mutation (N1493C/C1670S) was inserted into full-length rVWF (rVWF-CC) and VWF-N1515Q (VWF-N1515Q-CC). This mutation creates a structurally constrained A2 due to the presence of a long-range disulfide bridge, homologous to those present in the A1 and A3 domains. (B) Clearance was assessed in VWF−/− mice. All results are plotted as percentage residual VWF:antigen levels relative to the amount injected. Data are presented as mean ± SEM.
Discussion
Although the biosynthesis, structure, and functional properties of VWF have been well characterized, the molecular mechanisms through which VWF is cleared remain poorly understood.2,14 However, accumulating data have demonstrated that macrophages play important roles in regulating VWF clearance in vivo,21-26 and a number of putative macrophage receptors for VWF have been identified.23,26,38 Critically, however, the specific regions of the VWF glycoprotein involved in modulating interactions with these different macrophage receptors have not been determined. In this study, using a series of in vivo and in vitro methodologies, we demonstrate that the A1-A2-A3 domains of VWF contain a receptor-recognition site important in mediating VWF binding to macrophages in vitro and in regulating VWF clearance by macrophages in vivo. Furthermore, studies using isolated recombinant A domains demonstrated that the A1 domain plays a critical role in regulating macrophage binding. This observation is in keeping with previous studies demonstrating that full-length VWF binding to macrophages is significantly enhanced in the presence of ristocetin, botrecetin, or shear stress.22,23,26 Importantly, however, our novel data further demonstrate that the ability of the isolated A1 domain to interact with macrophages is markedly attenuated when the A1 domain is linked to the other A domains (A1-A2-A3 truncation), suggesting that the receptor binding site may not be accessible in normal globular VWF.
Previous reports have demonstrated that desialylation of human VWF results in a marked reduction in plasma half-life.29,32-34 In addition, although the molecular mechanisms involved were not elucidated, enhanced VWF clearance has also been associated with loss of specific O-linked glycans.30,58 In this paper, we demonstrate that complete loss of N-linked glycan expression following PNGase F digestion also results in markedly enhanced macrophage-mediated VWF clearance. Interestingly, recent studies have reported that N-linked glycan expression on factor X also plays a key role in regulating interaction with macrophages.59 Although the molecular mechanism through which N-linked glycan determinants regulate coagulation glycoprotein clearance remains unknown, the effect may be due to general properties of the complex sugar chains or instead may be attributable to particular carbohydrate structures located at specific N-linked sites.42,43 Given the role of the A1-A2-A3 domains in modulating the interaction of VWF with macrophages, we further investigated whether the 2 N-linked glycosylation sites located at N1515 and N1574, respectively, might play a particular role in regulating VWF clearance. Importantly, we observed that loss of N-linked glycans following site-directed mutagenesis at either N1515 or N1574 resulted in markedly enhanced clearance of full-length rVWF. Furthermore, introduction of the N1515Q or N1574Q substitutions into the A1-A2-A3 fragment also resulted in significantly enhanced clearance. In addition, the enhanced clearance phenotypes observed with N1515Q and N1574Q in full-length VWF, and also in the truncated A1-A2-A3 fragment, were all significantly attenuated following clodronate-induced macrophage depletion. Altogether, these novel findings therefore demonstrate that the N-linked glycan structures within the A2 domain play an important role in protecting VWF against macrophage-mediated clearance in vivo. Moreover, the reduced survival of VWF observed following loss of the N-linked glycan structures in A2 is predominantly due to local effects within the A1-A2-A3 region triggering enhanced macrophage clearance.
Accumulating data suggest that the LRP1 receptor may play a key role in regulating macrophage binding and clearance of VWF.23,26 Furthermore, Wohner et al recently reported that the A1 domain of VWF (but not the isolated A2 or A3 domains) could bind to purified LRP1 in vitro.26 Consequently, we investigated whether the LRP1 receptor may be involved in modulating the enhanced clearance of VWF-N1515Q and VWF-N1574Q. Interestingly, the reduced survival of both VWF-N1515Q and VWF-N1574Q was significantly attenuated in the presence of RAP. These findings suggest that the N-linked glycan expressed at N1515 and N1574 play a critical role in protecting VWF against LRP1-mediated macrophage clearance. Although the molecular mechanism(s) through which these N-linked glycans regulate LRP1-mediated clearance remain unclear, previous studies have demonstrated that expression of carbohydrate determinants can directly influence glycoprotein interactions through either charge-mediated mechanisms, or by modifying glycoprotein conformation.54-57 Consequently, we hypothesize that the protective effect of the large complex N-linked glycan structures in the A2 domain may be due to steric hindrance, with covering of cryptic LRP1 binding sites. Alternatively, and perhaps more likely, variation in A2 domain carbohydrate structures may cause conformational changes that result in enhanced LRP1-modulated clearance. This hypothesis is supported by the observation that removal of the N-linked glycans at N1515 no longer enhances VWF clearance in the presence of a structurally constrained A2 domain. Although our findings demonstrate a critical role for LRP1 in modulating macrophage-mediated clearance of VWF glycoforms, it is important to consider that a number of other macrophage receptors can also bind VWF.38,39,60 Included among these macrophage receptors are the lectins ASGPR, Gal-1, and Gal-3. We observed that VWF binding to both galectins was significantly reduced following loss of N-linked glycans (data not shown). Moreover, ASGPR inhibition with asiolo-orosomucoid did not attenuate the enhanced in vivo clearance of VWF-N1515Q. Nevertheless, previous studies have shown that LRP1 can form heterologous functional complexes with a number of other macrophage receptors, including β2-integrins.60 Additional studies will be necessary to determine whether the N1515 and/or N1574 glycans influence VWF interactions with any of these other macrophage receptors in addition to LRP1.
Previous studies have demonstrated that VWF clearance occurs independently of ADAMTS13 proteolysis and is not influenced by VWF multimer size.52,61 Nonetheless, it is interesting that in addition to influencing VWF clearance by macrophages, the N-linked glycans expressed within the A2 domain of VWF have also been shown to modulate susceptibility to proteolysis by ADAMTS13.42,62 In particular, McKinnon et al showed that loss of the N-linked glycan at N1574 resulted in significantly enhanced VWF proteolysis by ADAMTS13.42 Furthermore, differential scanning fluorimetry has confirmed that glycosylation at N1574 plays an important role in stabilization of the A2 domain against unfolding.44 However, in contrast to its marked effect upon clearance, loss of the VWF N1515 glycan did not significantly influence susceptibility to ADAMTS13 cleavage and did not have any significant effect on the thermostability of the A2 domain.42,44 Further studies will be necessary to define the biological mechanisms through which the carbohydrate structures at N1515 and N1574 regulate macrophage-mediated clearance. However, it is interesting that ABO(H) blood group determinants, which influence both VWF proteolysis by ADAMTS13 and VWF clearance, are expressed on both of these complex N-linked glycans within the A2 domain of pd-VWF.7,42
In the normal circulation, VWF adopts a globular conformation, such that the glycoprotein Ibα (GPIbα) binding site in the VWF A1 domain remains largely hidden.63 However, exposure to mechanical shear stress results in unwinding of globular VWF. As part of this unfolding process, previous studies have described both uncoupling of the A1A2A3 tridomain cluster, as well as conformational changes within the individual A domains.64,65 Consequently the platelet binding site in A1 becomes exposed, and the ADAMTS13 cleavage site (Tyr1605-Met1606) that is buried within the A2 domain becomes accessible.64,66 Our data, together with those recently published from other groups,22,23,26 suggest that conformational changes in the VWF A domains lead to exposure of cryptic receptor binding sites within A1-A2-A3 that trigger macrophage clearance. Given the thrombotic potential of VWF in its active conformation, targeting of unfolded VWF for rapid macrophage-mediated clearance has biological plausibility. Our findings are also consistent with the hypothesis that specific mutations within the A1 domain result in enhanced clearance due to increased binding to both platelet Gp1bα and macrophage LRP1.67 Further studies will be required to define the roles played by LRP1 and other macrophage receptors in modulating the enhanced clearance phenotypes associated with type 1C mutations located in other VWF domains. Interestingly however, preliminary data suggest that several other independent regions of VWF (including D′D3 and D4) are also able to bind to LRP1.14
Presented in abstract form as an oral presentation at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 8 December 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Nico van Rooijen of the Foundation Clodronate Liposomes (Haarlem, The Netherlands) for generously providing the liposome-clodronate and Orla Rawley for technical assistance in expression studies.
M.L. and T.A.J.M. acknowledge support from the Imperial College Biomedical Research Centre. This work was supported by a Science Foundation Ireland Principal Investigator Award (11/PI/1066) (J.S.O.).
Authorship
Contribution: A.C., J.M.O., S.A., G.B., S.W., C.D., T.M.B., and T.A.J.M. performed experiments; A.C., J.M.O., P.G.F., T.M.B., R.J.S.P., M.L., T.A.J.M., and J.S.O. designed the research and analyzed the data; and all authors were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: M.L. has received speaker fees from Bayer, Octapharma, and Pfizer; advisory board fees from CSL-Behring, Pfizer, Bayer, and Grifols; and research support from Bayer and CSL Behring. J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, and Octapharma; served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, and Pfizer; and received research grant funding awards from Baxter, Bayer, Pfizer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: James S. O’Donnell, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, 123 St. Stephen’s Green, Dublin 2, Ireland; e-mail jamesodonnell@rcsi.ie.
References
Author notes
A.C. and J.M.O. contributed equally to this study.